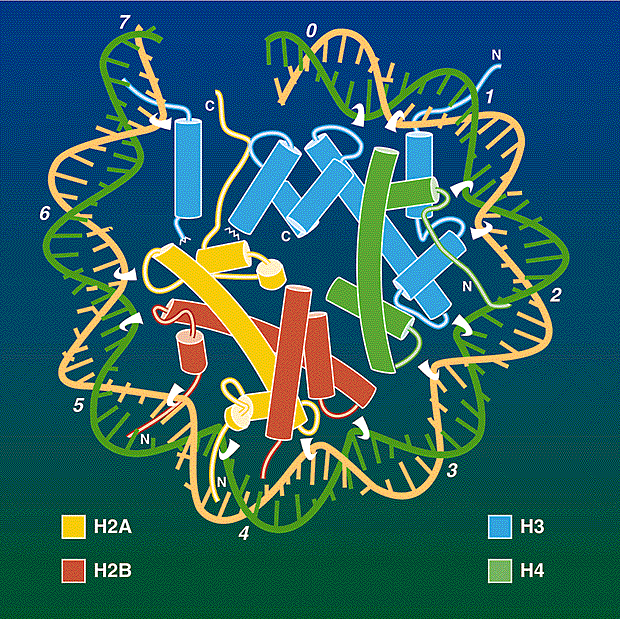
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Access through your institution Buy or subscribe For many years, biologists have been trying to understand how DNA is packaged into chromosomes. A breakthrough came in the 1970s when it was
established that all eukaryotic chromosomes consist of a regularly repeating protein-DNA complex called the nucleosome1. Each nucleosome consists of a protein octamer, made up of two copies
each of histones H2A, H2B, H3 and H4, which, together with the fifth histone, H1, organizes about 200 base pairs (bp) of DNA. Further organization involves the assembly of nucleosomes into
higher-order chromatin structures. On page 251of this issue, Luger _et al_.2 report the much awaited high-resolution structure of the nucleosome core particle. This landmark structure
confirms many of the expected features of the nucleosome core. But it also reveals some surprises. Importantly, it provides a structural basis for interpreting the many molecular mechanisms
that have evolved to regulate access to the DNA in chromatin. This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe
to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF
Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact
customer support REFERENCES * Kornberg, R. D. _Annu. Rev. Biochem_. 46, 931–954 (1977). Google Scholar * Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J.
_Nature_ 389, 251–260 (1997). Article ADS CAS Google Scholar * Richmond, T. J., Finch, J. T., Rushton, B., Rhodes, D. & Klug, A. _Nature_ 311, 532–537 (1984). Article ADS CAS
Google Scholar * Arents, G., Burlingame, R. W., Wang, B. C., Love, W. E. & Moudrianakis, E. N. _Proc. Natl Acad. Sci. USA_ 88, 10148–10152 (1991). Google Scholar * Burley, S. K., Xie,
X., Clark, K. L.& Shu, F. _Curr_. _Opin_. _Struct_. _Biol_. 7, 94-102 (1997). * Prunell, A. _et al._ _Science_ 204, 855–858 (1979). Google Scholar * Travers, A. A. _Trends Biochem.
Sci._ 12, 108–112 (1987). Google Scholar * Rhodes, D. & Klug, A. _Nature_ 286, 573–578 (1980). Article ADS CAS Google Scholar * Pryciak, P. M. & Varmus, H. E. _Cell_ 69, 769–780
(1992). Google Scholar * Turner, B. M. _Cell_ 75, 5–8 (1993). Google Scholar * Tskiyama, T. & Wu, C. _Curr. Opin. Genet. Dev._ 7, 182–191 (1997). Google Scholar * Wolffe, A. P.
_Nature_ 387, 16–17 (1997). Article ADS CAS Google Scholar * von Holde, K. & Zlatanova, J. _Proc. Natl Acad. Sci. USA_ 93, 10548–10555 (1996). Google Scholar * Hecht, A., Laroche,
T., Strahl-Bolsinger, S., Gasser, S. M. & Grunstein, M. _Cell_ 80, 583–592 (1995). Google Scholar * Finch, J. T. _et al. Nature_ 269, 29–36 (1977). Google Scholar Download references
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * MRC Laboratory of Molecular Biology, Hills Road, CB2 2QH, Cambridge<, UK Daniela Rhodes Authors * Daniela Rhodes View author publications You
can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Rhodes, D. The nucleosome core all wrapped up.
_Nature_ 389, 231–232 (1997). https://doi.org/10.1038/38386 Download citation * Issue Date: 18 September 1997 * DOI: https://doi.org/10.1038/38386 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative




