- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Access through your institution Buy or subscribe Newly synthesized proteins that leave the endoplasmic reticulum (ER) are funnelled through the Golgi complex before being sorted for
transport to their different final destinations. Traditional approaches have elucidated the biochemical requirements for such transport1,2,3 and have established a role for transport
intermediates4,5,6,7,8. New techniques for tagging proteins fluorescently9,10 have made it possible to follow the complete life history of single transport intermediates in living cells,
including their formation, path and velocity _en route_ to the Golgi complex. We have now visualized ER-to-Golgi transport using the viral glycoprotein ts045 VSVG tagged with green
fluorescent protein (VSVG-GFP). Upon export from the ER, VSVG-GFP became concentrated in many differently shaped, rapidly forming pre-Golgi structures, which translocated inwards towards the
Golgi complex along microtubules by using the microtubule minus-end-directed motor complex of dynein/dynactin. No loss of fluorescent material from pre-Golgi structures occurred during
their translocation to the Golgi complex and they frequently stretched into tubular shapes. Together, our results indicate that these pre-Golgi carrier structures moving unidirectionally
along microtubule tracks are responsible for transporting VSVG-GFP through the cytoplasm to the Golgi complex. This contrasts with the traditional focus on small vesicles as the primary
vehicles for ER-to-Golgi transport. The initial targets for vesicles budding from the ER _en route_ to the Golgi complex are pleiomorphic tubulovesicular structures found in the Golgi region
and in peripheral sites4,5,6,7,8, which are enriched in several proteins (including the COP1 subunit β-COP and ERGIC53)15,16,17,18, and whose proposed function is to concentrate and sort
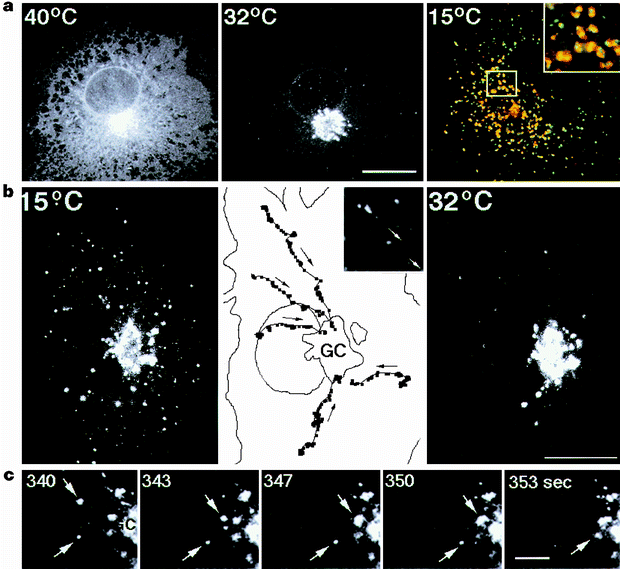
secretory cargo19,20. At reduced temperatures (such as 15 °C) these pre-Golgi structures become markedly enlarged and accumulate secretory products21, presumably owing to a rate-limiting
step in membrane transport through these intermediates22,23. VSVG-GFP accumulated in such intermediate structures upon incubation for 3 h at 15 °C, co-localizing extensively with β-COP (Fig.
1a; 15 °C) and ERGIC53 (data not shown). This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal
Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may
be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
REFERENCES * Rothman, J. E. & Wieland, F. T. Protein sorting by transport vesicles. _Science_ 272, 227–234 (1996). Google Scholar * Schekman, R. & Orci, L. Coat proteins and vesicle
budding. _Science_ 271, 1526–1533 (1996). Google Scholar * Aridor, M., Bannykh, S., Rowe, T. & Balch, W. E. Sequential coupling between CopII and CopI vesicle coats in endoplasmic
reticulum to Golgi transport. _J. Cell Biol._ 131, 1–19 (1995). Google Scholar * Pluttner, H., Davidson, H. W., Saraste, J. & Balch, W. E. Morphological analysis of protein transport
from the ER to Golgi membranes in digitonin-permeabilized cells: role of the p58 containing compartment. _J. Cell Biol._ 119, 1097–1116 (1992). Google Scholar * Saraste, J. & Svensson,
K. Distribution of the intermediate elements operating in ER to Golgi transport. _J. Cell Sci._ 100, 415–430 (1991). Google Scholar * Saraste, J. & Kuismanen, E. Pathways of protein
sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. _Semin. Cell Biol._ 3, 343–355 (1992). Google Scholar * 7. Krijnse-Locker, J., Ericsson, M.,
Rottier, P. J. & Griffiths, G. Characterization of the budding compartment of mouse hepatitis virus: Evidence that transport from the RER to the Golgi complex requires only one vesicular
transport step. _J. Cell Biol._ 124, 55–70 (1994). Google Scholar * Stinchcombe, J. C., Nomoto, H., Cutler, D. F. & Hopkins, C. R. Anterograde and retrograde traffic between the rough
endoplasmic reticulum and the Golgi complex. _J. Cell Biol._ 131, 1387–1401 (1995). Google Scholar * Prasher, D. C., Eckenrode, V. K., Ward, W. W., Prendergast, F. G. & Cormier, M. J.
Primary structure of the Aequorea victoria green-fluorescent protein. _Gene_ 111, 229–233 (1992). Google Scholar * Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W. & Prasher, D. C.
Green fluorescent protein as a marker for gene expression. _Science_ 263, 802–805 (1994). Google Scholar * Kreis, T. E. & Lodish, H. F. Oligomerization is essential for transport of
vesicular stomatitis viral glycoprotein to the cell surface. _Cell_ 46, 929–937 (1986). Google Scholar * Beckers, C. J., Keller, D. S. & Balch, W. E. Semi-intact cells permeable to
macromolecules: Use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. _Cell_ 50, 523–534 (1987). Google Scholar * Bergmann, J. E. Using
temperature-sensitive mutants of VSV to study membrane protein biogenesis. _Methods Cell Biol._ 32, 85–110 (1989). Google Scholar * Cole, N. B., Sciaky, N., Marotta, A., Song, J. &
Lippincott-Schwartz. J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. _Mol. Biol. Cell_ 7, 631–650 (1996).
Article CAS Google Scholar * Schweizer, A., Fransen, J. A. M., Bachi, T., Ginsel, L. & Hauri, H. -P. Identification, by a monoclonal antibody, of a 53 kD protein associated with a
tubulo-vesicular compartment at the cis-side of the Golgi apparatus. _J. Cell Biol._ 107, 1643–1653 (1988). Google Scholar * Lippincott-Schwartz, J., Cole, N. B., Marotta, A., Conrad, P. A.
& Bloom, G. S. Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic. _J. Cell Biol._ 128, 293–306 (1995). Google Scholar * Pepperkok, R._et al_. βCOP is essential
for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex _in vivo_. _Cell_ 74, 71–82 (1993). Google Scholar * Peter, F., Plutner, H., Zhu, H., Kreis, T. E.
& Balch, W. E. β-COP is essential for transport of protein from the endoplasmic reticulum to the Golgi _in vitro_. _J. Cell Biol._ 122, 1155–1168 (1993). Google Scholar * Balch, W. E.,
McCaffery, J. M., Pluttner, H. & Farquhar, M. G. Vesicular stomatitis virus is sorted and concentrated upon exit from the endoplasmic reticulum. _Cell_ 76, 841–852 (1994). Google Scholar
* Bannykh, S. I., Rowe, T. & Balch, W. E. The organization of endoplasmic reticulum export complexes. _J. Cell Biol._ 135, 19–35 (1996). Google Scholar * Kuismanen, E. & Saraste,
J. Low temperature-induced transport blocks as tools to manipulate membrane traffic. _Methods Cell Biol._ 32, 257–274 (1989). Google Scholar * Hauri, H. -P. & Schweizer, A. The
endoplasmic reticulum–Golgi intermediate compartment. _Curr. Opin. Cell Biol._ 4, 600–608 (1992). Google Scholar * Lotti, L. V., Torrisi, M. R., Pascale, M. C. & Bonatti, S.
Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the Golgi complex. _J. Cell Biol._ 118, 43–50 (1992). Google
Scholar * Walker, R. A. & Sheetz, M. P. Cytoplasmic microtubule-associated motors. _Annu. Rev. Biochem._ 62, 429–451 (1993). Google Scholar * Cole, N. B._et al_. Diffusional mobility
of Golgi proteins in membranes of living cells. _Science_ 273, 797–801 (1996). Google Scholar * Schroer, T. A., Bingham, J. B. & Gill, S. R. Actin-related protein 1 and cytoplasmic
dynein-based motility: What's the connection? _Trends Cell Biol._ 6, 212–215 (1996). Google Scholar * Gaglio, T._et al_. Opposing motor activities are required for the organization of
the mammalian mitotic spindle pole. _J. Cell Biol._ 135, 399–414 (1996). Google Scholar * Echeverri, C. J., Paschal, B. B., Vaughan, K. T. & Vallee, R. B. Molecular characterization of
the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. _J. Cell Biol._ 132, 617–633 (1996). Google Scholar *
Burkhardt, J. K., Echeverri, C. J. & Vallee, R. B. Overexpression of the p50 subunit of dynactin perturbs the positioning of the Golgi apparatus and endosomes. _Mol. Biol. Cell_ 6, 266a
(1995). Google Scholar * Gallione, C. J. & Rose, J. K. Asingle amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral
glycoprotein. _J. Virol._ 54, 374–382 (1985). Google Scholar * Vaisberg, E. A., Grissom, P. M. & McIntosh, J. R. Mammalian cells express three distinct dynein heavy chains that are
localized to different cytoplasmic organelles. _J. Cell Biol._ 133, 831–842 (1996). Google Scholar Download references ACKNOWLEDGEMENTS We thank R. Klausner, E. Siggia, J. Bonifacino, C.
Smith, J. Donaldson, J. Ellenberg and R. Stearman for valuable comments and suggestions, and J. Rose for his generous gift of reagent. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Cell
Biology and Metabolism Branch, National Institute of Child Health and Human Development, Building 18T, NICHD, NIH, Bethesda, 20892, Maryland, USA John F. Presley, Nelson B. Cole, Koret
Hirschberg, Kristien J. M. Zaal & Jennifer Lippincott-Schwartz * Department of Biology, The Johns Hopkins University, Baltimore, 21218, Maryland, USA Trina A. Schroer Authors * John F.
Presley View author publications You can also search for this author inPubMed Google Scholar * Nelson B. Cole View author publications You can also search for this author inPubMed Google
Scholar * Trina A. Schroer View author publications You can also search for this author inPubMed Google Scholar * Koret Hirschberg View author publications You can also search for this
author inPubMed Google Scholar * Kristien J. M. Zaal View author publications You can also search for this author inPubMed Google Scholar * Jennifer Lippincott-Schwartz View author
publications You can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Presley, J., Cole, N., Schroer,
T. _et al._ ER-to-Golgi transport visualized in living cells. _Nature_ 389, 81–85 (1997). https://doi.org/10.1038/38001 Download citation * Received: 21 March 1997 * Accepted: 16 June 1997 *
Issue Date: 04 September 1997 * DOI: https://doi.org/10.1038/38001 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative

