- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
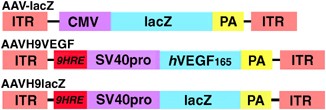
Uncontrolled expression of vascular endothelial growth factor (VEGF) in vivo may cause unexpected side effects, such as brain hemangioma or tumor growth. Because hypoxia-inducible factor-1
(HIF-1) is upregulated during cerebral ischemia and regulates gene expression by binding to a cis-acting hypoxia-responsive element (HRE), we therefore used a novel HRE, originating in the
3′-end of the erythropoietin (Epo) gene, to control gene expression in the ischemic brain. A concatemer of nine copies (H9) of the consensus sequence of HRE was used to mediate hypoxia
induction. Three groups of adult CD-1 mice received AAVH9-VEGF, AAVH9-lacZ or saline injection, and then underwent 45 min of transient middle cerebral artery occlusion (tMCAO). Results show
that HIF-1 was persistently expressed in the ischemic brain. VEGF was overexpressed in the ischemic perifocal region in AAVH9-VEGF-transduced mice. Double-labeled immunostaining showed that
VEGF expressed in neurons and astrocytes but not endothelial cells, suggesting that adeno-associated virus (AAV) vectors transduced neurons and astrocytes predominantly. The total number of
microvessels/enlarged microvessels was greatly increased in the AAVH9-VEGF-transduced mice (180±29/27±4) compared to the AAVH9-lacZ (118±19/14±3) or saline-treated (119±20/14±2) mice after
tMCAO (P