- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
You have full access to this article via your institution. Download PDF In the last few years, adeno-associated vectors (AAV) have generated significant interest as safe and effective
vehicles for gene transfer. In the cardiovascular field, there has been considerable enthusiasm for AAV because of their small size, which allows them to pass through vessel walls and to
reach the myocardium, and for their promise of a relatively long-term payload gene expression. However, there have been two reported examples of dose-dependent generation of CD8+ T-cell
responses to AAV capsid proteins in humans, limiting the therapeutic potential of otherwise promising therapies for hemophilia1 and type I hypertriglyceridemia deficiency2. The study by
McTiernan _et al._3 in this issue comparing the effects of gene transfer of type 2 AAV (AAV2) encoding TNFRII-Fc with empty AAV2 capsids may provide some clue to new pathways controlling the
delicate balance between induction of deleterious T-cell responses and operational immune tolerance to the AAV2 and payload gene proteins. These experiments in the baboon were initially
performed as pre-clinical safety studies of AAV2 TNFRII-Fc gene therapy as a therapy for heart failure. Although the potential value of this approach as heart-failure therapy has since been
reconsidered based on other results, the observations by McTiernan _et al._3 that myocarditis resulted following the injection of AAV2 encoding TNFRII-Fc but not AAV2 empty capsids in the
heart of baboons may have broad implications for AAV-based gene therapy. The authors attempt to sort through three reasons why myocarditis might occur: * 1 immune response directed toward
AAV capsids; * 2 immune response directed toward the transgene: TNFRII-Fc; and * 3 biological response induced by TNFRII-Fc. Overall, this is an interesting and difficult study performed in
a preclinical setting for therapeutics (TNFRII-Fc). The authors stumble on a surprising finding, and with the limited number of baboons they conduct a number of serological and histological
measurements in an attempt to explain the findings. Their results seem to rule out an immune response directed toward the transgene: TNFRII-Fc. However, based on the data provided, we do not
agree with their conclusion suggesting that myocarditis represents a general phenomenon of cellular responses to _all_ AAV vectors encoding foreign proteins. An alternative explanation is
that the combination of anti-TNF coding transgenes and AAV results in cellular immune responses against AAV capsid proteins _that would not occur otherwise_. This alternative hypothesis is
supported by the following: * 1) AAV vectors delivering the transgene for the sarcoplasmic reticulum ATPase2a have been studied in sheep, pig and primates, with no evidence of myocarditis,
T-cell infiltrates or damage to cardiac tissue, as assessed by histopathological analysis or highly sensitive assays for cardiac enzymes.4, 5 * 2) Operational _tolerance_ to the product of
foreign transgenes following _in vivo_ gene transfer of AAV vectors is associated with CD4+ T-cell tolerance involving anergy and deletion mechanisms. This has been demonstrated for Factor
IX in primates6 and ovalbumin in mice.7 In both cases, the critical role of CD4+CD25+ T regulatory cells has been demonstrated. * 3) Latency induced by viral infections are attributed to the
generation of CD4+CD25+ T regulatory cells,8 and the critical role of TNF in the generation of such T regulatory cells has recently been elucidated,9 suggesting a biological link in the
study by McTiernan _et al._3 between anti-TNF therapy and the generation of myocarditis. * 4) Experimental support for our alternative hypothesis in primates is provided in the study by
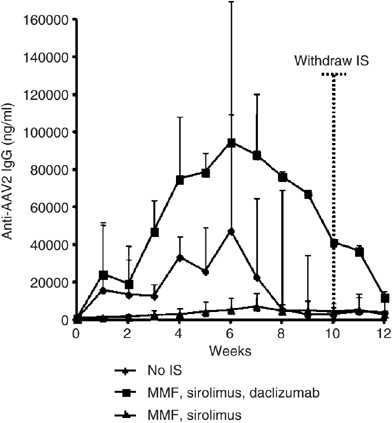
Mingozzi _et al._,6 where generation of humoral immune responses was studied when immunosuppressive agents were combined with an AAV2-hF.IX vector (Figure 1).6 Antibody responses against
AAV2 capsid proteins were significantly blunted with the administration of mycophenolate mofetil (MMF) and sirolimus; however, when daclizumab (a humanized anti-CD25 antibody) was added to
this regimen, the anti-AAV2 antibody response was _higher than in non-immunosuppressed animals._ This suggests that a CD4+CD25+ T regulatory pathway is active in the operational tolerance
documented to AAV capsid proteins. Therefore, the biological consequence of locally inhibiting TNF signaling may have resulted in myocarditis in the study by McTiernan _et al._3 In this
alternative model, local inhibition of TNF prevents the generation of tolerogenic anti-AAV capsid CD4+CD25+ T cells with subsequent _enhancement_ of a cellular immune response against the
AAV capsid antigens. In other words, blocking TNF (and subsequent prevention of T regulatory cell development) may have been enough to tip the balance of an immune response to AAV vector
triggered initially by local innate immunity toward a destructive cellular immune response and myocarditis. Our overall conclusions also differ from those of the authors. We do not believe
that these data are discouraging to the field of AAV gene therapy. Rather, we are encouraged by these findings, as they potentially provide clues for further manipulating the immune system
with modalities that may enhance the success of AAV therapeutics. For example, the use of agents such as sirolimus may be of benefit as this drug has been shown to promote the induction of
regulatory T cells.10, 11, 12 Finally, recent studies in animal models have shown that adoptive transfer of CD4+CD25+ T regulatory cells can prevent or even cure allergic and autoimmune
diseases and the cells appear to induce transplantation tolerance.13 Thus, we hope that these new data by McTiernan _et al._3 stimulate new thinking regarding the immunology of AAV vectors
and the critical importance of understanding the possible effect due to the biological activities of payload genes on the local and systemic environments. REFERENCES * Manno CS, Pierce GF,
Arruda VR, Glader B, Ragni M, Rasko JJ _et al_. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. _Nat Med_ 2006; 12:
342–347. Article CAS Google Scholar * Mingozzi F, Muelenberg J, Hui D, Basner-Tschakarajn E, de Pos P, High KA . Intramuscular Administration of an AAV1 Vector in Humans Results in Capsid
Specific T cell Responses. _Mol Ther_ 2007; 15 (Suppl 1): 3400, #1056. Google Scholar * McTiernan CF, Mathier MA, Zhu X, Xiao X, Klein K, Swan CH _et al_. Myocarditis following
adeno-associated viral expression of human soluble TNF receptor (TNFRII-Fc) in baboon hearts. _Gene Therapy_ 2007. * Preovolos AC, Mennen MT, Bilney A, Mariani J, Kaye DM, Power JM .
Development of a novel perfusion technique to allow targeted delivery of gene therapy—the V-Focus system. _J Extra Corpor Technol_ 2006; 38: 51–52. PubMed PubMed Central Google Scholar *
Ly HQ, Kawase Y, Prunier F, Lebeche D, Shi Y, Yoneyama R _et al_. Cardiac function improvement following _in vivo_ intracaoroanry adeno-associated virus type 1 vector gene transfer of
SERCA2a in a pre-clinical model of heart failure. _Circulation_ 2007; 116: 321. Article Google Scholar * Mingozzi F, Hasbrouck NC, Basner-Tschkarajan E, Edmonson SA, Hui DJ, Sabatino DE
_et al_. Modulation of tolerance to the transgene product in a non-human primate model of AAV-mediated gene transfer to liver. _Blood_ 2007; 110: 2334–2341. Article CAS Google Scholar *
Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C _et al_. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic _in vivo_ gene
transfer. _Blood_ 2007; 110: 1132–1140. Article CAS Google Scholar * Raghavan S, Holmgren J . CD4+CD25+ suppressor T cells regulate pathogen induced inflammation and disease. _FEMS
Immunol Med Microbiol_ 2005; 44: 121–127. Article CAS Google Scholar * Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ . Interaction of TNF with TNF receptor type 2 promotes
expansion and function of mouse CD4+CD25+ T regulatory cells. _J Immunol_ 2007; 179: 154–161. Article CAS Google Scholar * Battaglia M, Stabilini A, Roncarolo MG . Rapamycin selectively
expands CD4+CD25+FoxP3+ regulatory T cells. _Blood_ 2005; 105: 4743–4748. Article CAS Google Scholar * Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG .
Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. _J Immunol_ 2006; 177: 8338–8347. Article CAS Google
Scholar * Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E _et al_. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate
antigen-specific transplantation tolerance. _Diabetes_ 2006; 55: 40–49. Article CAS Google Scholar * Jiang S, Lechler RI . CD4+CD25+ regulatory T-cell therapy for allergy, autoimmune
disease and transplant rejection. _Inflamm Allergy Drug Targets_ 2006; 5: 239–242. Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Cardiovascular Research Center, Mount Sinai School of Medicine, New York, NY, USA R J Hajjar * Celladon Corporation, La Jolla, CA, USA K Zsebo Authors * R J Hajjar View author publications
You can also search for this author inPubMed Google Scholar * K Zsebo View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence
to K Zsebo. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hajjar, R., Zsebo, K. AAV vectors and cardiovascular disease: Targeting TNF receptor in the
heart: clue to way forward with AAV?. _Gene Ther_ 14, 1611–1612 (2007). https://doi.org/10.1038/sj.gt.3303047 Download citation * Published: 18 October 2007 * Issue Date: December 2007 *
DOI: https://doi.org/10.1038/sj.gt.3303047 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative