- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Hematopoietic stem cells in the bone marrow (BM) give rise to all blood cells. According to the classic model of hematopoiesis, the differentiation paths leading to the myeloid and
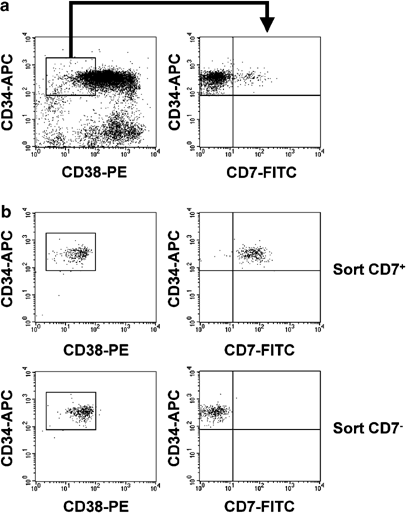
lymphoid lineages segregate early. A candidate ‘common lymphoid progenitor’ (CLP) has been isolated from CD34+CD38− human cord blood cells based on CD7 expression. Here, we confirm the B-
and NK-differentiation potential of CD34+CD38−CD7+ cells and show in addition that this population has strong capacity to differentiate into T cells. As CD34+CD38−CD7+ cells are virtually
devoid of myeloid differentiation potential, these cells represent true CLPs. To unravel the molecular mechanisms underlying lymphoid commitment, we performed genome-wide gene expression
profiling on sorted CD34+CD38−CD7+ and CD34+CD38−CD7− cells. Interestingly, lymphoid-affiliated genes were mainly upregulated in the CD7+ population, while myeloid-specific genes were
downregulated. This supports the hypothesis that lineage commitment is accompanied by the shutdown of inappropriate gene expression and the upregulation of lineage-specific genes. In
addition, we identified several highly expressed genes that have not been described in hematopoiesis before. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only
$21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A NEW LYMPHOID-PRIMED
PROGENITOR MARKED BY _DACH1_ DOWNREGULATION IDENTIFIED WITH SINGLE CELL MULTI-OMICS Article 19 October 2020 TERMINAL DEOXYNUCLEOTIDYL TRANSFERASE AND CD84 IDENTIFY HUMAN MULTI-POTENT
LYMPHOID PROGENITORS Article Open access 13 July 2024 CHARACTERIZATION AND GENERATION OF HUMAN DEFINITIVE MULTIPOTENT HEMATOPOIETIC STEM/PROGENITOR CELLS Article Open access 01 December 2020
REFERENCES * Kondo M, Weissman IL, Akashi K . Identification of clonogenic common lymphoid progenitors in mouse bone marrow. _Cell_ 1997; 91: 661–672. Article CAS Google Scholar * Akashi
K, Traver D, Miyamoto T, Weissman IL . A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. _Nature_ 2000; 404: 193–197. Article CAS Google Scholar * Allman D,
Sambandam A, Kim S, Miller JP, Pagan A, Well D _et al_. Thymopoiesis independent of common lymphoid progenitors. _Nat Immunol_ 2003; 4: 168–174. Article CAS Google Scholar * Schwarz BA,
Bhandoola A . Circulating hematopoietic progenitors with T lineage potential. _Nat Immunol_ 2004; 5: 953–960. Article CAS Google Scholar * Katsura Y . Redefinition of lymphoid
progenitors. _Nat Rev Immunol_ 2002; 2: 127–132. Article CAS Google Scholar * Galy A, Travis M, Cen D, Chen B . Human T, B, natural killer, and dendritic cells arise from a common bone
marrow progenitor cell subset. _Immunity_ 1995; 3: 459–473. Article CAS Google Scholar * Ishii T, Nishihara M, Ma F, Ebihara Y, Tsuji K, Asano S _et al_. Expression of stromal
cell-derived factor-1/pre-B cell growth-stimulating factor receptor, CXC chemokine receptor 4, on CD34+ human bone marrow cells is a phenotypic alteration for committed lymphoid progenitors.
_J Immunol_ 1999; 163: 3612–3620. CAS PubMed Google Scholar * Manz MG, Miyamoto T, Akashi K, Weissman IL . Prospective isolation of human clonogenic common myeloid progenitors. _Proc
Natl Acad Sci USA_ 2002; 99: 11872–11877. Article CAS Google Scholar * Hao Q-L, Zhu J, Price MA, Payne KJ, Barsky LW, Crooks GM . Identification of a novel, human multilymphoid progenitor
in cord blood. _Blood_ 2001; 97: 3683–3690. Article CAS Google Scholar * Haddad R, Guardiola P, Izac B, Thibault C, Radich J, Delezoide A-L _et al_. Molecular characterization of early
human T/NK and B-lymphoid progenitor cells in umbilical cord blood. _Blood_ 2004; 104: 3918–3926. Article CAS Google Scholar * Robin C, Pflumio F, Vainchenker W, Coulombel L .
Identification of lymphomyeloid primitive progenitor cells in fresh human cord blood and in the marrow of nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice transplanted with
human CD34+ cord blood cells. _J Exp Med_ 1999; 189: 1601–1610. Article CAS Google Scholar * Dik WA, Pike-Overzet K, Weerkamp F, de Ridder D, de Haas EFE, Baert MRM _et al_. New insights
on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. _J Exp Med_ 2005; 201: 1715–1723. Article CAS Google Scholar *
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U _et al_. Exploration, normalization, and summaries of high density oligonucleotide array probe level data.
_Biostatistics_ 2003; 4: 249–264. Article Google Scholar * Khatri P, Draghici S, Ostermeier GC, Krawetz SA . Profiling gene expression using Onto-Express. _Genomics_ 2002; 79: 266–270.
Article CAS Google Scholar * van Zelm MC, van der Burg M, de Ridder D, Barendregt BH, de Haas EFE, Reinders MJT _et al_. Ig gene rearrangement steps are initiated in early human precursor
B cell subsets and correlate with specific transcription factor expression. _J Immunol_ 2005; 175: 5912–5922. Article CAS Google Scholar * Livak KJ, Schmittgen TD . Analysis of relative
gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. _Methods_ 2001; 25: 402–408. Article CAS Google Scholar * De Smedt M, Reynvoet K, Kerre T, Taghon T,
Verhasselt B, Vandekerckhove B _et al_. Active form of Notch imposes T cell fate in human progenitor cells. _J Immunol_ 2002; 169: 3021–3029. Article CAS Google Scholar * Nemeth MJ,
Curtis DJ, Kirby MR, Garrett-Beal LJ, Seidel NE, Cline AP _et al_. Hmgb3: an HMG-box family member expressed in primitive hematopoietic cells that inhibits myeloid and B-cell
differentiation. _Blood_ 2003; 102: 1298–1306. Article CAS Google Scholar * Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL . Differential expression of novel
potential regulators in hematopoietic stem cells. _PLos Genet_ 2005; 1: e28. Article Google Scholar * Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I _et al_. Regulation of
oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. _Nature_ 2004; 431: 997–1002. Article CAS Google Scholar * Bortoluzzi S, d'Alessi F, Romualdi C,
Danieli GA . Differential expression of genes coding for ribosomal proteins in different human tissues. _Bioinformatics_ 2001; 17: 1152–1157. Article CAS Google Scholar * Baklouti F,
Huang SC, Tang TK, Delaunay J, Marchesi VT, Benz EJJ . Asynchronous regulation of splicing events within protein 4.1 pre-mRNA during erythroid differentiation. _Blood_ 1996; 87: 3934–3941.
CAS PubMed Google Scholar * Stoss O, Olbrich M, Hartmann AM, Konig H, Memmott J, Andreadis A _et al_. The STAR/GSG family protein rSLM-2 regulates the selection of alternative splice
sites. _J Biol Chem_ 2001; 276: 8665–8673. Article CAS Google Scholar * Levesque J-P, Simmons PJ . Cytoskeleton and integrin-mediated adhesion signaling in human CD34+ hemopoietic
progenitor cells. _Exp Hematol_ 1999; 27: 579–586. Article CAS Google Scholar * Juliano RL . Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins,
cadherins, selectins, and immunoglobulin-superfamily members. _Annu Rev Pharmacol Toxicol_ 2002; 42: 283–323. Article CAS Google Scholar * Janmey PA . The cytoskeleton and cell signaling:
component localization and mechanical coupling. _Physiol Rev_ 1998; 78: 763–781. Article CAS Google Scholar * Doherty FJ, Dawson S, Mayer RJ . The ubiquitin-proteasome pathway of
intracellular proteolysis. _Essays Biochem_ 2002; 38: 51–63. Article CAS Google Scholar * Akashi K, He X, Chen J, Iwasaki H, Niu C, Steenhard B _et al_. Transcriptional accessibility for
genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. _Blood_ 2003; 101: 383–389. Article CAS Google Scholar * Miyamoto T, Iwasaki
H, Reizis B, Ye M, Graf T, Weissman IL _et al_. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. _Dev Cell_ 2002; 3: 137–147. Article CAS Google
Scholar * Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C _et al_. Multilineage gene expression precedes commitment in the hemopoietic system. _Genes Dev_ 1997; 11: 774–785.
Article CAS Google Scholar * Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R _et al_. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell.
_Cell_ 2001; 105: 369–377. Article CAS Google Scholar * Haddad R, Guimiot F, Six E, Jourquin F, Setterblad N, Kahn E _et al_. Dynamics of thymus-colonizing cells during human
development. _Immunity_ 2006; 24: 217–230. Article CAS Google Scholar * Weerkamp F, Baert MR, Brugman MH, Dik WA, de Haas EF, Visser TP _et al_. The human thymus contains multipotent
progenitors with T/B-lymphoid, myeloid and erythroid lineage potential. _Blood_ 2005; 107: 3131–3137. Article Google Scholar * Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM,
Zúñiga-Pflücker JC, Petrie HT . Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. _Immunity_ 2004; 20:
735–745. Article CAS Google Scholar * Weerkamp F, Pike-Overzet K, Staal FJ . T-sing progenitors to commit. _Trends Immunol_ 2006; 27: 125–131. Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS We thank the staff of the Bloedtransfusiecentrum Oost-Vlaanderen for the supply of umbilical cord blood samples and Marie-José De Bosscher for lymphoprepping. We
are grateful to Dick De Ridder for help with microarray analysis and to Inge Van de Walle for excellent technical assistance. This work was supported by grants from the Fund for Scientific
Research Flanders (Belgium) and from the Ghent University concerted research program. AUTHOR INFORMATION Author notes * I Hoebeke Present address: I Hoebeke, Diabetes Research Center,
Brussels Free University (VUB), Laarbeeklaan 103, B-1090, Brussels, Belgium * F Stolz Present address: Laboratory of Molecular Cell Biology, Institute of Botany and Microbiology, Katholieke
Universiteit Leuven, AUTHORS AND AFFILIATIONS * Department of Clinical Chemistry, Microbiology and Immunology, Ghent University Hospital, Ghent, Belgium I Hoebeke, M De Smedt, F Stolz, J
Plum & G Leclercq * Department of Immunology, Erasmus University Medical Center, Rotterdam, The Netherlands K Pike-Overzet & F J T Staal * Department of Molecular Microbiology,
Flanders Interuniversity Institute of Biotechnology (VIB), Leuven, Belgium F Stolz Authors * I Hoebeke View author publications You can also search for this author inPubMed Google Scholar *
M De Smedt View author publications You can also search for this author inPubMed Google Scholar * F Stolz View author publications You can also search for this author inPubMed Google Scholar
* K Pike-Overzet View author publications You can also search for this author inPubMed Google Scholar * F J T Staal View author publications You can also search for this author inPubMed
Google Scholar * J Plum View author publications You can also search for this author inPubMed Google Scholar * G Leclercq View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to G Leclercq. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Leukemia website
(http://www.nature.com/leu) SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE S1 (DOC 299 KB) SUPPLEMENTARY TABLES S2 (DOC 191 KB) SUPPLEMENTARY TABLES S3 (DOC 203 KB) SUPPLEMENTARY REFERENCES
CITED IN TABLES S2 AND S3 (DOC 106 KB) SUPPLEMENTARY TABLE S4 (DOC 22 KB) SUPPLEMENTARY TABLE S5 (DOC 75 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Hoebeke, I., De Smedt, M., Stolz, F. _et al._ T-, B- and NK-lymphoid, but not myeloid cells arise from human CD34+CD38−CD7+ common lymphoid progenitors expressing lymphoid-specific
genes. _Leukemia_ 21, 311–319 (2007). https://doi.org/10.1038/sj.leu.2404488 Download citation * Received: 31 August 2006 * Accepted: 19 October 2006 * Published: 14 December 2006 * Issue
Date: 01 February 2007 * DOI: https://doi.org/10.1038/sj.leu.2404488 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * human * hematopoiesis * stem
cells * cord blood * lymphoid progenitor






