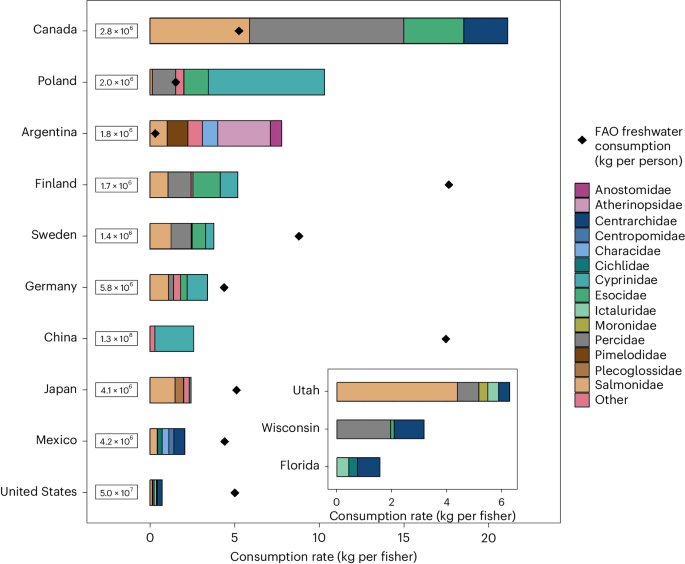
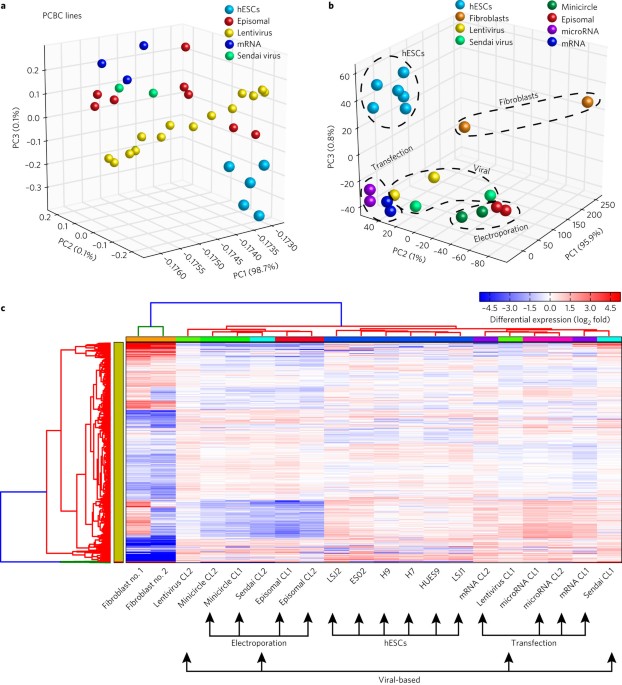
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT THE 2,4-dinitrophenylhydrazones of keto-acids are frequently used to identify these metabolic intermediates. The clear statement by Clift and Cook1 regarding the ready thermal
decarboxylation of the 2,4-dinitrophenylhydrazones of oxaloacetic and acetoacetic acids has sometimes been overlooked, particularly in the use of melting points as a means of identification.
Some of our observations bear on the problem. SIMILAR CONTENT BEING VIEWED BY OTHERS A WAY TO THIOACETATE ESTERS COMPATIBLE WITH NON-OXIDATIVE PREBIOTIC CONDITIONS Article Open access 02
September 2020 PREBIOTIC FORMATION OF THIOESTERS VIA CYCLIC ANHYDRIDES AS A KEY STEP IN THE EMERGENCE OF METABOLISM Article Open access 27 February 2025 FORMATION OF THIOESTERS BY
DEHYDROGENATIVE COUPLING OF THIOLS AND ALCOHOLS WITH H2 EVOLUTION Article 28 September 2020 ARTICLE PDF REFERENCES * Clift, F. P., and Cook, R. P., _Biochem. J._, 26, 1800 (1932). Article
CAS Google Scholar * Moriwaki, T., Katsuki, H., and Tanaka, S., _J. Chem. Soc. Japan_, 76, 1367 (1955). CAS Google Scholar * Metzler, D. E., Olivard, J., and Snell, E. E., _J. Amer.
Chem. Soc._, 76, 644 (1954). Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Chemistry and Biochemistry, Institutum Divi Thomae,
Cincinnati, Ohio AMBROSE M. TOKUSHIGE & ELTON S. COOK Authors * AMBROSE M. TOKUSHIGE View author publications You can also search for this author inPubMed Google Scholar * ELTON S. COOK
View author publications You can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE TOKUSHIGE, A., COOK,
E. Thermal Decarboxylation of Some Keto-Acid Hydrazones. _Nature_ 184, 267 (1959). https://doi.org/10.1038/184267a0 Download citation * Issue Date: 25 July 1959 * DOI:
https://doi.org/10.1038/184267a0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative