- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
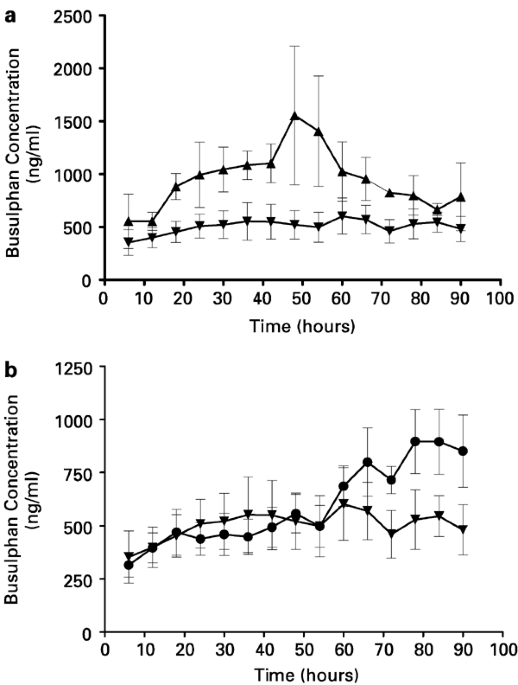
SUMMARY: Busulfan (Bu) is an important component of some myeloablative regimens prior to stem cell transplantation (SCT). Over the last few years it has been shown that other drugs
administered concomitantly can influence Bu pharmacokinetics. In the present study, we compared Bu concentrations (trough levels) in three groups of patients. Group A (_n_=5) received
metronidazole as graft-versus-host disease prophylaxis during Bu treatment. Group B (_n_=9) received Bu only for 2 days followed by 2 days of Bu and metronidazole. Group C (_n_=10) was a
control group that received Bu without metronidazole. The mean Bu levels for Group A receiving metronidazole during conditioning was significantly (_P_<0.001) higher (948±280 ng/ml),
compared to those observed in the control group (507±75 ng/ml). In Group B, the administration of metronidazole resulted in a significant (_P_<0.001) increase in Bu levels (807±90 ng/ml)
during the last 2 days, compared to 452±68 ng/ml during the first 2 days. In Group A, one patient died with multiorgan failure, three experienced veno-occlusive disease (VOD) and one
developed hemorrhagic cystitis. Elevated liver transaminases (AST, ALT) and bilirubin were detected in all Group A patients. In Group B, six patients had elevated liver function tests but no
VOD was observed. We conclude that metronidazole should not be administered simultaneously with Bu to avoid the high plasma levels of Bu, which may lead to severe toxicity and/or treatment
related mortality. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS IMPACT OF BUSULFAN PHARMACOKINETICS ON OUTCOME IN ADULT PATIENTS RECEIVING AN ALLOGENEIC HEMATOPOIETIC CELL TRANSPLANTATION
Article Open access 31 March 2022 A PRACTICAL GUIDE TO THERAPEUTIC DRUG MONITORING IN BUSULFAN: RECOMMENDATIONS FROM THE PHARMACIST COMMITTEE OF THE EUROPEAN SOCIETY FOR BLOOD AND MARROW
TRANSPLANTATION (EBMT) Article 13 September 2024 CLINICAL OUTCOMES OF INDIVIDUALIZED BUSULFAN-DOSING IN HEMATOPOIETIC STEM CELL TRANSPLANTATION IN CHINESE CHILDREN UNDERGOING WITH
THERAPEUTIC DRUG MONITORING Article 17 January 2022 REFERENCES * Socie G, Clift RA, Blaise D et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide
before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. _Blood_ 2001; 98: 3569–3574. Article CAS PubMed Google Scholar * Bolinger AM, Zangwill
AB, Slattery JT et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. _Bone Marrow
Transplant_ 2000; 25: 925–930. Article CAS PubMed Google Scholar * Hassan M, Ljungman P, Bolme P et al. Busulfan bioavailability. _Blood_ 1994; 84: 2144–2150. CAS PubMed Google Scholar
* Vassal G, Fischer A, Challine D et al. Busulfan disposition below the age of three: alteration in children with lysosomal storage disease. _Blood_ 1993; 82: 1030–1034. CAS PubMed
Google Scholar * Vassal G, Deroussent A, Challine D et al. Is 600 mg/m2 the appropriate dosage of busulfan in children undergoing bone marrow transplantation? _Blood_ 1992; 79: 2475–2479.
CAS PubMed Google Scholar * Hassan M, Oberg G, Bjorkholm M et al. Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. _Cancer Chemother Pharmacol_ 1993; 33:
181–186. Article CAS PubMed Google Scholar * Buggia I, Zecca M, Alessandrino EP et al. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow
transplantation. GITMO (Gruppo Italiano Trapianto di Midollo Osseo). _Anticancer Res_ 1996; 16: 2083–2088. CAS PubMed Google Scholar * Hassan M, Svensson JO, Nilsson C et al. Ketobemidone
may alter busulfan pharmacokinetics during high-dose therapy. _Ther Drug Monit_ 2000; 22: 383–385. Article CAS PubMed Google Scholar * Grochow LB . Busulfan disposition: the role of
therapeutic monitoring in bone marrow transplantation induction regimens. _Semin Oncol_ 1993; 20: 18–25; quiz 26. CAS PubMed Google Scholar * Vassal G, Koscielny S, Challine D et al.
Busulfan disposition and hepatic veno-occlusive disease in children undergoing bone marrow transplantation. _Cancer Chemother Pharmacol_ 1996; 37: 247–253. Article CAS PubMed Google
Scholar * Vergnon JM, Boucheron S, Riffat J et al. Interstitial pneumopathies caused by busulfan. Histologic, developmental and bronchoalveolar lavage analysis of 3 cases. _Rev Med Intern_
1988; 9: 377–383. Article CAS Google Scholar * Ljungman P, Hassan M, Bekassy AN et al. Busulfan concentration in relation to permanent alopecia in recipients of bone marrow transplants.
_Bone Marrow Transplant_ 1995; 15: 869–871. CAS PubMed Google Scholar * Chattergoon DS, Saunders EF, Klein J et al. An improved limited sampling method for individualised busulphan dosing
in bone marrow transplantation in children. _Bone Marrow Transplant_ 1997; 20: 347–354. Article CAS PubMed Google Scholar * Hassan M, Fasth A, Gerritsen B et al. Busulphan kinetics and
limited sampling model in children with leukemia and inherited disorders. _Bone Marrow Transplant_ 1996; 18: 843–850. CAS PubMed Google Scholar * Zander AR, Zabelina T, Kroger N et al.
Use of a five-agent GVHD prevention regimen in recipients of unrelated donor marrow. _Bone Marrow Transplant_ 1999; 23: 889–893. Article CAS PubMed Google Scholar * Beelen DW, Elmaagacli
A, Muller KD et al. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease
after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. _Blood_ 1999; 93: 3267–3275. CAS
PubMed Google Scholar * Samuelson J . Why metronidazole is active against both bacteria and parasites. _Antimicrob Agents Chemother_ 1999; 43: 1533–1541. Article CAS PubMed PubMed
Central Google Scholar * Storb R, Deeg HJ, Whitehead J et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow
trans-plantation for leukemia. _N Engl J Med_ 1986; 314: 729–735. Article CAS PubMed Google Scholar * Ringden O, Remberger M, Persson U et al. Similar incidence of graft-versus-host
disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings. _Bone Marrow Transplant_ 1995; 15: 619–625. CAS PubMed Google Scholar * Hassan M,
Ehrsson H . Gas chromatographic determination of busulfan in plasma with electron-capture detection. _J Chromatogr_ 1983; 277: 374–380. Article CAS PubMed Google Scholar * Jones RJ, Lee
KS, Beschorner WE et al. Venoocclusive disease of the liver following bone marrow transplantation. _Transplantation_ 1987; 44: 778–783. Article CAS PubMed Google Scholar * Vassal G,
Deroussent A, Hartmann O et al. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. _Cancer Res_ 1990; 50: 6203–6207. CAS PubMed Google
Scholar * Hassan Z, Ljungman P, Ringden O et al. Pharmacokinetics of liposomal busulphan in man. _Bone Marrow Transplant_ 2001; 27: 479–485. Article CAS PubMed Google Scholar *
Olavarria E, Hassan M, Eades A et al. A phase I/II study of multiple-dose intravenous busulfan as myeloablation prior to stem cell transplantation. _Leukemia_ 2000; 14: 1954–1959. Article
CAS PubMed Google Scholar * Andersson BS, Madden T, Tran HT et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as
pretransplantation conditioning therapy: a phase I study. _Biol Blood Marrow Transplant_ 2000; 6: 548–554. Article CAS PubMed Google Scholar * Schuler US, Renner UD, Kroschinsky F et al.
Intravenous busulphan for conditioning before autologous or allogeneic human blood stem cell transplantation. _Br J Haematol_ 2001; 114: 944–950. Article CAS PubMed Google Scholar *
Loft S . Metronidazole and antipyrine as probes for the study of foreign compound metabolism. _Pharmacol Toxicol_ 1990; 66: 1–31. Article CAS PubMed Google Scholar * Levy RH . Cytochrome
_P_450 isozymes and antiepileptic drug interactions. _Epilepsia_ 1995; 36: S8–S13. Article PubMed Google Scholar * Loft S, Poulsen HE . Metabolism of metronidazole and antipyrine in
isolated rat hepatocytes. Influence of sex and enzyme induction and inhibition. _Biochem Pharmacol_ 1989; 38: 1125–1136. Article CAS PubMed Google Scholar * Kazmier FJ . A significant
interaction between metronidazole and warfarin. _Mayo Clin Proc_ 1976; 51: 782–784. CAS PubMed Google Scholar * van der Weide J, Steijns LS, van Weelden MJ et al. The effect of genetic
polymorphism of cytochrome _P_450 CYP2C9 on phenytoin dose requirement. _Pharmacogenetics_ 2001; 11: 287–291. Article CAS PubMed Google Scholar * Spina E, Pisani F, Perucca E .
Clinically significant pharmacokinetic drug interactions with carbamazepine. An update. _Clin Pharmacokinet_ 1996; 31: 198–214. Article CAS PubMed Google Scholar * Hassan M, Ehrsson H .
Metabolism of 14C-busulfan in isolated perfused rat liver. _Eur J Drug Metab Pharmacokinet_ 1987; 12: 71–76. Article CAS PubMed Google Scholar * Marchand DH, Remmel RP, Abdel-Monem MM .
Biliary excretion of a glutathione conjugate of busulfan and 1,4-diiodobutane in the rat. _Drug Metab Dispos_ 1988; 16: 85–92. CAS PubMed Google Scholar * Gibbs JP, Yang JS, Slattery JT .
Comparison of human liver and small intestinal glutathione _S_-transferase-catalyzed busulfan conjugation _in vitro_. _Drug Metab Dispos_ 1998; 26: 52–55. CAS PubMed Google Scholar *
Gibbs JP, Murray G, Risler L et al. Age-dependent tetrahydrothiophenium ion formation in young children and adults receiving high-dose busulfan. _Cancer Res_ 1997; 57: 5509–5516. CAS PubMed
Google Scholar * Hassan M, Oberg G, Ehrsson H et al. Pharmacokinetic and metabolic studies of high-dose busulphan in adults. _Eur J Clin Pharmacol_ 1989; 36: 525–530. Article CAS PubMed
Google Scholar * Hassan M, Ehrsson H . Urinary metabolites of busulfan in the rat. _Drug Metab Dispos_ 1987; 15: 399–402. CAS PubMed Google Scholar * Damani LA, Houdi AA . Cytochrome
_P_-450 and FAD-monooxygenase mediated S- and N-oxygenations. _Drug Metabol Drug Interact_ 1988; 6: 235–244. CAS PubMed Google Scholar * Sherratt AJ, Banet DE, Linder MW et al.
Potentiation of 3-methylcholanthrene induction of rat hepatic cytochrome P450IA1 by dexamethasone _in vivo_. _J Pharmacol Exp Ther_ 1989; 249: 667–672. CAS PubMed Google Scholar * Larsson
P, Cybulski W, Tjalve H . Binding of 3H-metronidazole in olfactory, respiratory and alimentary epithelia in rats. _Pharmacol Toxicol_ 1997; 81: 65–73. Article CAS PubMed Google Scholar
* Martelli A, Allavena A, Robbiano L et al. Comparison of the sensitivity of human and rat hepatocytes to the geno-toxic effects of metronidazole. _Pharmacol Toxicol_ 1990; 66: 329–334.
Article CAS PubMed Google Scholar * Hassan M, Ljungman P, Ringden O et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval
influence on therapeutic efficacy and therapy-related toxicity. _Bone Marrow Transplant_ 2000; 25: 915–924. Article CAS PubMed Google Scholar * Shulman HM, Luk K, Deeg HJ et al.
Induction of hepatic veno-occlusive disease in dogs. _Am J Pathol_ 1987; 126: 114–125. CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The authors express
their gratitude to the Swedish Children Cancer Society (BCF) grant no. PROJ 01/059, King Gustav V Jubilee fund grant no. 00:510 and Stockholms Cancer Foundation grant no. 02:119. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Division of Hematology, Department of Medicine, Laboratory of Hematology, Huddinge University Hospital, Stockholm, Sweden C Nilsson, P Ljungman & M
Hassan * Center for Allogeneic Stem Cell Transplantation, Huddinge University Hospital, Stockholm, Sweden J Aschan, P Hentschke & O Ringdén Authors * C Nilsson View author publications
You can also search for this author inPubMed Google Scholar * J Aschan View author publications You can also search for this author inPubMed Google Scholar * P Hentschke View author
publications You can also search for this author inPubMed Google Scholar * O Ringdén View author publications You can also search for this author inPubMed Google Scholar * P Ljungman View
author publications You can also search for this author inPubMed Google Scholar * M Hassan View author publications You can also search for this author inPubMed Google Scholar RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nilsson, C., Aschan, J., Hentschke, P. _et al._ The effect of metronidazole on busulfan pharmacokinetics in patients
undergoing hematopoietic stem cell transplantation. _Bone Marrow Transplant_ 31, 429–435 (2003). https://doi.org/10.1038/sj.bmt.1703896 Download citation * Received: 14 March 2002 *
Accepted: 20 October 2002 * Published: 28 March 2003 * Issue Date: 01 March 2003 * DOI: https://doi.org/10.1038/sj.bmt.1703896 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * busulfan * pharmacokinetics * metronidazole * SCT * toxicity * drug interaction

:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)






