- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Sex differences in biological substrates of drug use and addiction are poorly understood. The present study investigated sexual dimorphisms in motor behavior following acute cocaine
administration (10, 20, or 40 mg/kg, i.p.). Cocaine increased stereotypy rating, horizontal and vertical activity in both sexes, and effects were always greater in females than males. A
population analysis using data from multiple experiments indicated that horizontal activity scores were normally distributed in males but not in females. Gonadectomy induced disparate
effects on cocaine-stimulated motor behavior. Population analysis indicated that castrated males exhibited more horizontal activity and stereotypy than shams. Ovariectomy did not affect
cocaine-stimulated stereotypy but did attenuate horizontal activity in a subset of rats that had not been vaginally lavaged. In summary, gonadectomy effects were sex and behavioral
topography specific and indicate that activational effects of gonadal hormones partially mediate the robust sex differences in cocaine-stimulated open-field behavior. SIMILAR CONTENT BEING
VIEWED BY OTHERS SEX AND STRAIN DIFFERENCES IN DYNAMIC AND STATIC PROPERTIES OF THE MESOLIMBIC DOPAMINE SYSTEM Article 14 July 2020 HORMONAL MILIEU DRIVES ECONOMIC DEMAND FOR COCAINE IN
FEMALE RATS Article 25 March 2022 SEX DIFFERENCES IN DOPAMINE RELEASE REGULATION IN THE STRIATUM Article 14 December 2020 MAIN Little is known about sex differences in biologic substrates of
drug taking and addiction. However, an emerging literature documents that sex differences in the behavioral responses to addictive drugs exist in humans. A sex difference in human affective
responses to cocaine has been reported in one study in which females reported more nervousness than males following intranasal cocaine (Kosten et al. 1996). Lukas and coworkers (1996)
showed that cocaine induced similar cardiovascular and affective responses in men and women, although plasma concentrations of cocaine were lower in women. In a laboratory setting, cocaine
cues induced more drug craving in female than male addicts (Robbins et al. 1999). These results suggest that women may be more sensitive to cocaine than men. In fact, women begin using
cocaine and enter treatment at earlier ages than men (Griffin et al. 1989; Mendelson et al. 1991) and have more severe cocaine use at intake than men (Kosten et al. 1993). Sex differences in
spontaneous and drug-stimulated behavior have been carefully studied in animals. Probably the most robust sex difference in behavior is the observation that female rats exhibit more running
wheel activity than males (for review see Beatty 1979). Female rats are also often reported to be more active than males in open-field situations (Beatty 1979). These sex differences in
basal or non-stimulated behavior are likely related to the reported sex differences in open-field behavior following psychomotor stimulant administration (van Hartesveldt and Joyce 1986).
Amphetamine is particularly well known for producing greater behavioral effects in female rats following acute administration (Schneider and Norton 1979; Savageau and Beatty 1981; Becker et
al. 1982; Camp et al. 1986; Camp and Robinson 1988a). Acute cocaine administration has also been reported to induce greater behavioral effects in female rats (van Haaren and Meyer 1991;
Haney et al. 1994; Bowman and Kuhn 1996) and in mice (Sershen et al. 1998). However, in other reports, sex differences in acute cocaine-stimulated motor behavior were not observed in naïve
Sprague-Dawley rats (Craft and Stratman 1996) and in three other rat strains (Cailhol and Mormede 1999). Thus, better evidence of sex-differences in acute behavioral effects in intact
animals exists for amphetamine than cocaine. Gonadal and especially ovarian hormones have been investigated as likely modulators of these sex differences in behavior. Ovarian hormone effects
on stimulant responses are complex, as ovariectomy and estrous cycle studies sometimes provide apparently contradictory results (van Hartesveldt and Joyce 1986). Two studies (Savageau and
Beatty 1981; Camp and Robinson 1988b) found that ovariectomy did not affect amphetamine-stimulated stereotypy, while only one showed effects on locomotion (Savageau and Beatty 1981). Despite
this absence of ovariectomy effect on stereotypy, the intensity of stereotyped behavior following amphetamine was greater during estrus (Becker and Cha 1989). Female rats in estrus also
make more rotations following amphetamine (Becker and Beer 1986) or electrical stimulation of the medial forebrain bundle (Robinson et al. 1981, 1982) than ovariectomized rats. Cocaine
effects on locomotion and stereotypy are also greater in female rats in estrus (Quinones-Jenab et al. 1999). Orchidectomy effects on behavioral responses to stimulants are even less
understood. Camp and Robinson (1988b) and Beatty et al. (1982) reported that castration increased amphetamine-induced stereotypy but not locomotion. A consistent picture about the role of
gonadal steroids on behavioral responses to stimulants has been elusive. Different behavioral strategies and paradigms used by different authors and the absence of males in many studies have
complicated the issue. A better description of biologic sex differences in acute cocaine-stimulated behavior is vital to understanding individual vulnerabilities in the establishment of
drug-taking because the degree of “liking” of the initial cocaine exposure is significantly associated with subsequent cocaine taking habits (Haertzen et al. 1983). Therefore, we conducted
the present studies to compare directly the effects of male and female gonadectomy on sex differences in acute cocaine-stimulated open-field behavior. This work examined multiple components
of motor activity in the open field: horizontal and vertical movements and integrated observational ratings of behavioral activation. Pooling data from multiple experiments to form
population databases led to novel observations of sex differences in individual responsiveness to cocaine. METHODS SUBJECTS Adult male and female Sprague-Dawley rats were purchased from
Charles River Laboratories (Raleigh, NC). They were segregated by sex and were housed in plastic cages under a 12:12 light: dark cycle with lights on at 0600 hr. Food and water were provided
_ad libitum_. The animal supplier performed bilateral ovariectomy, castration or sham surgery when rats were 60 days of age. Rats were tested two to three weeks after shipment on average.
Animals were moved to the testing facility and weighed the day before observations. Animal care was in accordance with the _Guide for the Care and Use of Laboratory Animals_ (NIH publication
865–23, Bethesda, MD) and approved by the Institutional Animal Care and Use Committee. ESTROUS CYCLE MONITORING Estrous cycle stage was monitored in several experiments (see Experiments
section) by analysis of cell types in vaginal lavages. Daily vaginal lavages were collected in the morning for at least eight consecutive days prior to testing and allowed to dry on
microscope slides. Slides were then fixed with ethanol and stained with toluidine blue. Identification of cell types was made microscopically according to published methods (Long and Evans
1922). Lavage was used to verify lack of cycling in ovariectomized rats in certain experiments (Cooper et al. 1993). BEHAVIORAL APPARATUS Initial studies of sex differences and gonadectomy
in cocaine effects used Opto-Varimex photocell chambers (Columbus Instruments, Columbus, OH). Later experiments were performed in similar photocell devices from a different supplier (San
Diego Instruments, Inc., San Diego, CA). The two devices were comprised of open plexiglass arenas with wood chip bedding on the floor. Horizontal activity (locomotion) and vertical activity
(rearing) were determined from interruptions of photobeams spaced one inch apart in both devices. Photocell interruptions were also recorded at 5 min intervals. Data from the later devices
were recorded on a computer using software from the manufacturer. Vertical activity is reported as the number of photocell interruptions. The horizontal distance traveled in inches is
reported as horizontal activity. BEHAVIORAL METHODS Assignment to test chambers was counterbalanced across and within days with respect to sex, cocaine dose, and surgical status. Experiments
were conducted only during the light cycle from 0900 to 1500 hours. Rats were allowed to habituate to the chambers for 40 minutes. Cocaine HCl was prepared fresh in 0.9% saline and either
the saline vehicle (1 ml/kg) or cocaine was injected i.p. In the initial dose response and gonadectomy experiments, the topography of behavior was assessed simultaneously with locomotor
activity by recording the occurrence of inactivity, rearing, grooming, locomotion, sniffing, continuous sniffing, and stereotypy during three observation periods consisting of fifteen
seconds each, every 5 min, beginning 5 min after dosing. Stereotypy included head weaving or bobbing, patterned locomotion, paw treading, and dyskinesia. A single observer, blind to the drug
treatment, watched all the rats in individual experiments. For each of the three observation periods, a summed stereotypy score modified from that of Gifford and Johnson (1992) was assigned
according to the behaviors observed. These three scores were then averaged to obtain a score for that minute. The scoring system was: 1, inactive; 2, grooming or locomotion or sniffing or
rearing; 3, sniffing with locomotion and/or rearing, or continuous sniffing; 4, continuous sniffing with continuous motion; 5, frequent stereotyped movements with locomotion; 6, almost
continuous stereotyped movements, restricted to one place in the cage. EXPERIMENTS The effects of sex and gonadal hormones on cocaine-stimulated behavior were examined in several
experiments. The initial experiment used naïve, intact males and intact, non-lavaged females. Horizontal motor activity (locomotion) was determined in the Opto-Varimex devices following i.p.
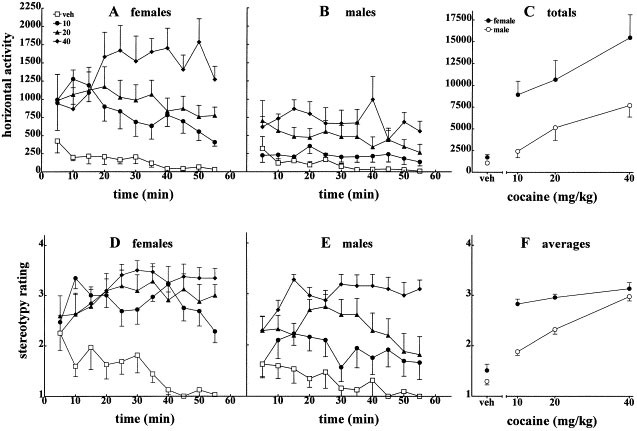
injection of either the saline vehicle, 10, 20, or 40 mg/kg cocaine. Simultaneously, these animals were observed to determine stereotypy ratings (Figure 1 ). We replicated this initial dose
response experiment in different activity monitoring devices that included both horizontal and vertical photobeam arrays (Figure 2). Castration of adult male and female rats was directly
compared in a dose-response experiment (Figure 3 ). Castrated and sham-castrated male and female rats of the same age were injected with either the saline vehicle, 10, 20, or 40 mg/kg
cocaine and horizontal and vertical motor activity was recorded simultaneous with observerrated stereotypy. All the horizontal activity data resulting from vehicle and 10 mg/kg cocaine
treatments from 19 different experiments were combined for a population-style analysis. Eleven of the 19 experiments included both male and female rats, five used only females, and three
used only males. Six of the 19 total experiments included sham-operated and gonadectomized females and males. In addition to these six experiments, three included ovariectomized and
sham-ovariectomized rats exclusively, and one used only castrated and sham-castrated males. The nine experiments that compared the effects of 10 mg/kg cocaine in sham and ovariectomized
females were subsequently categorized according to whether or not the rats had been vaginally lavaged. Cocaine dose-response relationships were determined for stereotypy and horizontal
activity in sham and castrated males in three experiments and these were combined to examine castration effects in a population. REAGENTS Cocaine HCl was obtained from NIDA and the Research
Triangle Institute (Research Triangle Park, NC). Cocaine solutions were prepared fresh daily in 0.9% saline. DATA ANALYSIS Motor activity (horizontal and vertical) and stereotypy data are
shown as the mean + SEM in 5-min intervals beginning immediately after injection. Activity session totals and average stereotypy scores of all 5 min intervals were also calculated and shown.
The effects of cocaine and sex on motor behavior and stereotypy were analyzed using two-way ANOVA with repeated measures on trial (five minute intervals in Figures 1 and 2: A, B, D, E). The
effects of sex, castration and cocaine were determined using three-way ANOVA (sex × surgery × dose) with repeated measures on trial (Figure 3). Repeated measures ANOVA used
Geisser-Greenhouse adjustments of probability levels for F-tests. ANOVA's were performed using NCSS 2000 software (NCSS, Inc, Kaysville, UT). Scattergrams and relative frequency
histograms were produced from populations of horizontal activity values using Prism 3.0 (Graphpad Software, Inc., Carlsbad, CA). This software was also used to test for deviations from
Gaussian distribution using the Kolmogorov-Smirnov test. Distributions were said to be significantly non-Gaussian when _p_ < .05. Sex and gonadectomy effects in populations were analyzed
individually using Student's T-test. When distributions were found to be significantly non-Gaussian the non-parametric Mann-Whitney test was used. Differences were considered
significant when _p_ < .05. RESULTS ACUTE BEHAVIORAL EFFECTS OF COCAINE IN INTACT RATS Cocaine increased horizontal activity in all rats and these effects were greater in female rats.
Figure 1 shows time course (A, B) and session totals (C) for horizontal activity. Horizontal activity in vehicle-treated rats gradually decreased or habituated throughout consecutive 5-min
intervals of the test session (Figure 1: A and B). The sex difference in cocaine-stimulated activity, though apparent over the entire dose range, is most clear at the lowest dose, 10 mg/kg.
At this dose locomotor stimulation relative to the vehicle treatment was marked in females and negligible in males. Two-way ANOVA indicated significant main effects on horizontal activity
for cocaine (F(3,56) = 15.6, _p_ < .0001) and sex (F(1, 56) = 22.4, _p_ = .001) but the dose by sex interaction was not significant (_p_ = .11). Horizontal activity varied across the test
session as indicated by the significant effect of the repeated factor, trial (F(10,560) = 2.85, _p_ = .002). Figures 1D, E, and F show that stereotypy ratings were greater in female rats
(F(1, 56) = 16.2, _p_ = .001). Sex differences again were most apparent for stereotypy at the low dose of cocaine (Figure 1F). Cocaine increased stereotypy rating in a dose-related fashion
in males, and at the highest dose males were comparable to females. Few rats of either sex transitioned into intense stereotyped behavior. Two-way ANOVA indicated that cocaine significantly
increased stereotypy rating (F(3, 56) = 33.1, _p_ < .001), but no interaction of dose and sex was found (_p_ = .08). Trial also exerted a significant effect on stereotypy (F(10,560) =
3.33, _p_ < .001). The initial dose-response activity experiment was replicated in a different testing apparatus that provided both horizontal and vertical motor activity data. Time
course data (Figures 2A and B) and session totals (Figure 2C) show that cocaine increased horizontal activity overall (F(3,33) = 19.5, _p_ < .001). Females exhibited more horizontal
activity than males (F(1, 33) = 12.3, _p_ = .001) and in this experiment, a significant interaction of sex and dose was observed (F(3, 33) = 3.11, _p_ = .04). The effect of trial did not
reach statistical significance (_p_ = .09). Figure 2 (C, D, E) shows the effect of cocaine on rearing behavior in male and female rats. Cocaine increased rearing overall (F(3, 33) = 4.8, _p_
= .007) and females reared more than males (F(1, 33) = 3.95, _p_ = .05). No interaction of sex and dose was found (_p_ = .39). The effect of trial was significant for vertical activity
(F(11,363) = 5.27, _p_ < .001). EFFECT OF OVARIECTOMY AND CASTRATION Figure 3 shows cocaine dose-response effects in ovariectomized, castrated, and sham-gonadectomized rats. Cocaine
significantly increased all three measures of behavior in this experiment. Figure 3A indicates that cocaine induced dose-related increases in horizontal activity (F(3,144) = 51.3, _p_ <
.001) and that horizontal activity was greater in all female than all male rats (F(1, 144) = 6.79, _p_ = .01). Figure 3B shows that cocaine increased rearing behavior (F(3,139) = 5.59, _p_
< .001) although not in a dose-related manner like for horizontal activity. Rearing was also greater in female rats overall (F(1, 139) = 11.1, _p_ = .002). Figure 3C shows that cocaine
increased stereotypy scores in all rats (F(3, 139) = 19.3, _p_ = .001) and female rats had higher stereotypy scores (F(1, 139) = 37.9, _p_ < .001). The sex differences observed in the
prior experiments for horizontal activity, rearing, and stereotypy also existed between sham ovariectomized and sham castrated rats. Two-way ANOVA (sex × dose) indicated that female shams
exhibited more horizontal activity (F(1,88) = 6.6, _p_ = .02), vertical activity (F(1, 85) = 15.6, _p_ = .0002), and stereotypy (F(1,87) = 21.8, _p_ < .001) than male shams. Of the three
indices of open field behavior, horizontal activity was least affected by gonadectomy. Neither ovariectomy nor castration altered horizontal activity significantly. In contrast, gonadectomy
produced disparate effects in males and females with regard to both vertical activity and stereotypy. ANOVA indicated significant interactions of gonadectomy and sex for vertical activity
(F(1,139) = 8.63, _p_ = .004) and stereotypy (F(1,144) = 13.6, _p_ < .001). Rearing was less in ovariectomized relative to sham females (F(1, 87) = 5.5, _p_ = .02) and greater in
castrated rats relative to sham males (F(1,52) = 16.1, _p_ < .001). Stereotypy rating was higher in castrated than sham-castrated rats (F(1, 55) = 5.61, _p_ = .02), but ovariectomy did
not affect stereotypy score (_p_ = .28). POPULATION ANALYSIS OF SEX DIFFERENCES Data from all experiments using vehicle and 10 mg/kg cocaine were combined to examine further sex and
individual differences in behavioral responses. Horizontal activity values for all intact male and female rats used in 19 different experiments have been plotted in Figure 4 as scattergrams,
group averages, and relative histograms. This analysis included 246 male rats and 344 females. The scattergram in 4A shows that most horizontal activity values from males administered 10
mg/kg cocaine are under 7500 and densely clustered. Only a relatively few rats in this group exhibited activity values above 7500. In contrast, the cocaine-treated female values show a much
greater range and a significant fraction of the population is higher than the high responding males. Group averages confirm the trends observed in the cocaine scattergram and extend it to
the saline data also. Figure 4B indicates that activity in females following both saline and 10 mg/kg cocaine is greater than the respective male groups (_p's_ < .001 by Mann-Whitney
tests). The robust sex differences in horizontal activity shown in Figure 4B reflect significant sex differences in each individual experiment. Both relative frequency histograms revealed
similar distribution patterns in Figures 4C and D. Male horizontal activity values following injection of either saline or 10 mg/kg cocaine exhibited typical “bell-shaped” distributions. A
larger proportion of high responders was seen in both female populations. These high responding females significantly skewed both distributions of female activity so that they were not
normally distributed (_p_ = .05). POPULATION ANALYSIS OF OVARIECTOMY DATA Populations of horizontal activity scores from nine separate experiments using ovariectomized and
sham-ovariectomized females injected with 10 mg/kg cocaine were also analyzed. These activity values were segregated according to whether the rats had previously been vaginally lavaged and
are shown separately in each panel of Figure 5 . Ovariectomy did not alter cocaine-stimulated horizontal activity in five experiments using lavaged rats (t-tests, _p's_ = 034–0.94).
Three of four experiments that tested the effect of ovariectomy in non-lavaged rats reported that ovariectomy significantly decreased cocaine-stimulated horizontal activity (_p_ < .05).
The fourth experiment, in which the effect of ovariectomy did not reach statistical significance (_p_ = .092), had the smallest sample size (_n_ = 6) and therefore least power of these four.
No significant differences were found between any groups following vehicle treatment and therefore were not shown. A scattergram of all the cocaine-stimulated horizontal activity data from
these nine ovariectomy experiments is shown in Figure 5A. In the lavaged ovariectomized and sham females groups, a few “high responding” values (> 15,000) were widely dispersed. No
differences were obvious between these two columns of data and their distributions were very similar (Figure 5C). A larger fraction of high responding values from the non-lavaged sham rats
was tightly clustered between 15,000 and 20,000 (Figure 5A). In sharp contrast, only two individuals in the non-lavaged ovariectomized group were in the same range. Likewise, the
distribution of horizontal activity values from non-lavaged ovariectomized rats appeared to have a different shape than that recorded in the corresponding sham females (Figure 5D). Relative
distributions of cocaine responses in non-lavaged rats suggest that ovariectomy selectively attenuated a high responding sub-population as an additional 22% of the sham, relative to the
ovariectomized, population had high activity values (above the 14,000 bin; Figure 5D). Group averages of these data (Figure 5B) indicate that horizontal activity in non-lavaged
ovariectomized rats is less than non-lavaged shams (t = 2.89, df = 95, _p_ = .005). A significant decrease in horizontal activity in ovariectomized rats treated with cocaine was also
determined when lavaged and non-lavaged data were considered together (t = 2.85, df = 199, _p_ = .0048, combination bars not shown). Ovariectomy did not affect horizontal activity in lavaged
rats (_p_ = .2). POPULATION ANALYSIS OF CASTRATION DATA A population-style analysis was also performed to determine the effects of male castration on cocaine-stimulated behavior by
combining data from three experiments (including the single castration experiment previously described). Each investigated the effects of vehicle, 10, 20, and 40 mg/kg cocaine on horizontal
activity and behavioral activation in castrated and sham-operated males (_n_ = 25–27/group). Because these male populations of 10 mg/kg cocaine values were too small for distribution
analysis, the population analysis considered dose-response effects on both horizontal activity and stereotypy. These data were analyzed by two-factor ANOVA (surgery × dose) and are shown in
Figure 6 . The population analysis indicated that castrated rats ambulated more than sham males overall (F(1,199) = 5.78, _p_ = .02). Stereotypy scores from the castrated male population
were greater than the shams (F(3,199) = 3.99, _p_ = .05). Cocaine increased horizontal activity (F(3,199) = 90, _p_ < .001) and stereotypy (F(3,199) = 85, _p_ < .001) in both castrated
and sham-operated males. Unlike ovariectomy, castration did not significantly alter horizontal activity when the 10 mg/kg cocaine data were considered separately (t-test, _p_ = .4).
DISCUSSION The present study reports robust sex differences in open-field behavioral responses to acute cocaine administration in rats. Cocaine consistently increased motor activity and
stereotypy more in female than male rats. Gonadectomy studies indicated a complex relationship between organizational and activational effects of gonadal steroids in mediating these robust
sex differences. Of the three behavioral indices measured, horizontal activity was least affected by gonadectomy and vertical activity was most affected. Population analysis of gonadectomy
effects proved instrumental for detecting modest activational effects of gonadal hormones on horizontal activity and isolated a novel interaction of vaginal lavage and ovariectomy on
cocaine-stimulated activity. The present behavioral results are consistent with previous reports of enhanced responses of female rats to amphetamine (Robinson et al. 1980, 1982; Becker et
al. 1982). Although more amphetamine reaches the brain of female rats (Becker et al. 1982), amphetamine still induces greater locomotor responses in female rats after doses are adjusted to
produce the same brain concentrations (Robinson et al. 1980; Becker et al. 1982). In contrast to amphetamine, the present sex differences in cocaine effects are not explained by
pharmacokinetic differences. This laboratory previously reported that plasma and brain concentrations of cocaine are the same in male and female rats following i.p. injection (Bowman et al.
1999). Furthermore, the same study showed that ovariectomy and castration did not affect plasma and brain cocaine concentrations. The sex differences in behavioral responses to amphetamine
and cocaine suggest these effects may result from basic sex differences in their neural targets, most likely the dopamine system. Different behavioral topographies are mediated by different
brain regions, neurotransmitters, and interactions of neurotransmitters (Randrup and Munkvad 1974; Kelly 1977). Furthermore, different doses of stimulant drugs selectively induce certain
behavioral topographies (Sharp et al. 1987; Walker et al. 1990). The current work shows that ovarian hormones selectively modulate certain aspects of the sex differences in
cocaine-stimulated open-field behavior. Ovariectomy decreased vertical activity relative to female shams but did not affect horizontal activity or stereotypy in the single gonadectomy
experiment (Figure 3). However, in the population analysis of 10 mg/kg cocaine data (with greater statistical power), ovariectomy did decrease horizontal activity. The robust effects on
rearing and the more limited effects on horizontal activity suggest that ovarian hormones have varying effects on different components of motor behavior. The population analysis revealed an
important source of variability in detecting ovariectomy effects on horizontal activity. The attenuation of cocaine-stimulated horizontal activity in the whole population of ovariectomized
rats was due to a subset of animals that had not been vaginally lavaged. Cocaine-stimulated horizontal activity in ovariectomized, lavaged rats was not different than sham, lavaged rats.
Relative distributions of horizontal activity values in non-lavaged rats seemed to indicate ovariectomy selectively attenuated a high responding sub-population. An effect of ovariectomy on
cocaine-stimulated vertical activity (Figure 3) was detected suggesting a varying effect of lavage on different topographies. Although the mechanism for this effect of vaginocervical
stimulation is unknown, these findings suggest that the impact of lavage must be considered in future behavioral studies of psychostimulant effects in rats. The present results concur with
other reports showing that ovariectomy did not affect cocaine-stimulated stereotypy. van Luijtelaar et al. (1996) found that sham and ovariectomized females were equally likely to engage in
cocaine-stimulated stereotyped behavior and at a greater rate than intact but not castrated males. Additionally, Sircar and Kim (1999) found no difference in behavioral ratings between sham
and ovariectomized females in response to acute injection of 15 mg/kg cocaine. Camp and Robinson (1988b) have reported that ovariectomy did not affect stereotypy following acute
administration of amphetamine. Thus, unlike the neural substrate(s) that mediate(s) cocaine-stimulated hyperactivity, the substrate mediating cocaine-stimulated stereotypy does not respond
to loss of ovarian hormones. The possible effect of vaginal lavage on cocaine-stimulated stereotypy has not been addressed, but would appear to be minimal in light of these numerous
references showing a lack of ovariectomy effect on stimulant-induced activity. The effects of testicular hormones on cocaine-stimulated motor behavior are more straightforward than those of
ovarian hormones. Testosterone exerted a slight but consistent inhibitory effect on all cocaine-stimulated behaviors. In the single gonadectomy experiment (Figure 3) castration increased
vertical activity and stereotypy rating relative to male shams. Castration of males had a more modest effect than ovariectomy on horizontal activity. Although the individual experiment
showed no effect of castration on horizontal activity, the combination of all dose-response data indicated a main effect of castration on horizontal activity. Thus, it appears that
activational effects of testosterone are much more involved with vertical activity and stereotypy than horizontal activity, again suggesting differential involvement in this case, of
testosterone, on specific behavioral topographies. The present study, the first investigation of male gonadal hormone effects on cocaine-stimulated activity, is concordant with analogous
work with amphetamine. Forgie and Stewart (1994) have shown that castration increased the locomotor response to acute amphetamine, although the effect dissipated with repeated exposure.
Another study found no effect of castration on amphetamine-stimulated horizontal activity (Menniti and Baum 1981). Similar to the present results, Dluzen et al. (1986) found that castration
increased amphetamine-stimulated rearing behavior. Adult castration increased the duration of stereotyped behavior in response to injection of 5 mg/kg amphetamine (Beatty et al. 1982). This
same study found that exogenous testosterone decreased stereotypy in castrated males and ovariectomized females. Thus, these previous studies of amphetamine-stimulated behaviors are similar
to the present results with cocaine, in that adult castration increased stereotypy and vertical activity; effects on horizontal activity were more modest. The present results and others
suggest that activational effects of gonadal hormones explain part but not all of the sex differences in both basal and cocaine-stimulated activity. Gonadal hormones also appear to have
organizational effects on open field behaviors in rodents (Beatty 1979). Castration before 30 days of age increases ambulation and rearing to female levels (Scouten et al. 1975; Bengelloun
et al. 1976). Female rats injected with testosterone neonatally or at puberty exhibited less ambulation and rearing than oil treated females (Stevens and Goldstein 1983). Likewise,
ovariectomy of female rats at one or eight days of age, but not at 23 days or later, reduced activity of adult animals in the open field. Thus, both organizational and activational effects
of gonadal hormones play certain roles in mediating the robust sex differences in cocaine-stimulated open-field behavior described in the present studies. The high responding female
subpopulation described in the present work is reminiscent of a previous report of individual differences in amphetamine sensitization described in male rats (Robinson 1988). Hooks and
coworkers (1991) have reported that the most active male rats in a novel environment (high responders) exhibit more horizontal activity following cocaine administration. A high responder was
defined as having an activity score above the median. Our data clearly show that female populations had more high responders than males. These populations of horizontal activity values from
all intact female rats had significantly skewed distributions following saline and cocaine administration, suggesting that cocaine might have accentuated a predisposition to increased
horizontal activity inherent in females. We will investigate whether this predisposition for high responding is related to the estrous cycle. The three clusters of horizontal activity values
from sham, non-lavaged rats in Figure 5A tantalizingly suggest just such a distribution by estrous cycle stage. Many aspects of dopaminergic function are regulated by gonadal steroids (van
Hartesveldt and Joyce 1986) and could contribute to the observed sex difference in cocaine-stimulated behavior. Rivest et al. (1995) reported a sex difference in the density of dopamine
transporters with a greater Bmax in female than male rats. Estradiol administration increases dopamine receptor density over the course of several days (Hruska 1986; Hruska and Nowak 1988).
Physiological doses of estradiol rapidly shift striatal D2 receptors from high to low affinity states (Levesque and Di Paolo 1988; Di Paolo et al. 1988). Ovarian hormones increase striatal
dopamine turnover (Di Paolo et al. 1985) and dopamine release (van Hartesveldt and Joyce 1986; Dluzen and Ramirez 1990; Peris et al. 1991; McDermott 1993) and amphetamine-stimulated dopamine
release (Becker and Ramirez 1980; Becker and Beer 1986). This laboratory reported that the rates for both dopamine uptake and release in the striatum of naïve female rats are significantly
higher than in males (Walker et al. 2000). This result suggests that sex differences in extracellular dopamine regulation may mediate the observed sex differences in saline-stimulated
horizontal activity in our populations. Furthermore, different kinetics of extracellular dopamine regulation lead to differential disruption by cocaine, producing the increased behavioral
responses to cocaine in female rats (Walker and Kuhn 1997). We believe this is a plausible mechanism to explain sex differences in stimulant responsivity. In summary, the present results
show that females exhibit substantially greater motor responses to cocaine and that gonadal steroids contribute to these differences. The present studies attempted to define the relative
magnitudes of sex and gonadal hormone effects on cocaine-stimulated motor behavior in rats. An emerging clinical database suggests that stimulant addictions in men and women have different
natural histories and may require different therapies. Development of preventive and therapeutic strategies for drug abuse requires better understanding of sex differences in biologic
substrates of drug use and addiction. The present results show that both current gonadal steroid state and underlying sex differences influence behavioral responses to cocaine in the rat.
REFERENCES * Beatty WW . (1979): Gonadal hormones and sex differences in nonreproductive behaviors in rodents: Organizational and activational influences. _Hormones Behav_ 12: 112–163
Article CAS Google Scholar * Beatty WW, Dodge AM, Traylor KL . (1982): Stereotyped behavior elicited by amphetamine in the rat: Influences of the testes. _Pharmacol Biochem Behav_ 16:
565–568 Article CAS PubMed Google Scholar * Becker JB, Beer ME . (1986): The influence of estrogen on nigrostriatal dopamine activity: Behavioral and neurochemical evidence for both pre-
and postsynaptic components. _Behav Brain Res_ 19: 27–33 Article CAS PubMed Google Scholar * Becker JB, Cha JH . (1989): Estrous cycle-dependent variation in amphetamine-induced
behaviors and striatal dopamine release assessed with microdialysis. _Behav Brain Res_ 35: 117–125 CAS PubMed Google Scholar * Becker JB, Ramirez VD . (1980): Sex differences in the
amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. _Brain Res_ 204: 361–372 Article Google Scholar * Becker JB, Robinson TE, Lorenz KA . (1982): Sex
differences and estrous cycle variations in amphetamine- elicited rotational behavior. _Eur J Pharmacol_ 80: 65–72 Article CAS PubMed Google Scholar * Bengelloun WA, Nelson DJ, Zent HM,
Beatty WW . (1976): Behavior of male and female rats with septal lesions: Influence of prior gonadectomy. _Physiol Behav_ 16: 317–330 Article CAS PubMed Google Scholar * Bowman BP, Kuhn
CM . (1996): Age-related differences in the chronic and acute response to cocaine in the rat. _Dev Psychobiol_ 29: 597–611 Article CAS PubMed Google Scholar * Bowman BP, Vaughan SR,
Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM . (1999): Effects of sex and gonadectomy on cocaine metabolism in the rat. _J Pharmacol Exp Ther_ 290: 1316–1323 CAS PubMed
Google Scholar * Cailhol S, Mormede P . (1999): Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. _Brain Res_ 842: 200–205
Article CAS PubMed Google Scholar * Camp DM, Becker JB, Robinson TE . (1986): Sex differences in the effects of gonadectomy on amphetamine-induced rotational behavior in rats. _Behav
Neural Biol_ 46: 491–495 Article CAS PubMed Google Scholar * Camp DM, Robinson TE . (1988a): Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic
D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. _Behav Brain Res_ 30: 55–68 Article CAS PubMed Google Scholar * Camp DM, Robinson TE . (1988b):
Susceptibility to sensitization. II. The influence of gonadal hormones on enduring changes in brain monoamines and behavior produced by the repeated administration of d-amphetamine or
restraint stress. _Behav Brain Res_ 30: 69–88 Article CAS PubMed Google Scholar * Cooper RL, Goldman JM, Vandenbergh JG . (1993): Monitoring of estrus cyclicity in the laboratory rodent
by vaginal lavage. In Chapin RE, Heindel JJ (eds), Reproductive toxicology, Vol 3B. Methods in Toxicology, Female Reproductive Systems. Orlando, FL, Academic Press, pp 45–56 * Craft RM,
Stratman JA . (1996): Discriminative stimulus effects of cocaine in female versus male rats. _Drug Alcohol Depend_ 42: 27–37 Article CAS PubMed Google Scholar * Di Paolo T, Falardeau P,
Morissette M . (1988): Striatal D-2 dopamine agonist binding sites fluctuate during the rat estrous cycle. _Life Sci_ 43: 665–672 Article CAS PubMed Google Scholar * Di Paolo T,
Rouillard C, Bedard P . (1985): 17 beta-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. _Eur J Pharmacol_ 117: 197–203 Article CAS PubMed Google
Scholar * Dluzen DE, Green MA, Ramirez VD . (1986): The effect of hormonal condition on dose-dependent amphetamine-stimulated behaviors in the male rat. _Horm Behav_ 20: 1–6 Article CAS
PubMed Google Scholar * Dluzen DE, Ramirez VD . (1990): In vitro progesterone modulation of amphetamine-stimulated dopamine release from the corpus striatum of ovariectomized
estrogen-treated female rats: Response characteristics. _Brain Res_ 517: 117–122 Article CAS PubMed Google Scholar * Forgie ML, Stewart J . (1994): Six differences in the
locomotor-activating effects of amphetamine: Role of circulating testosterone in adulthood. _Physiol Behav_ 55: 639–644 Article CAS PubMed Google Scholar * Griffin ML, Weiss RD, Mirin
SM, Lange U . (1989): A comparison of male and female cocaine abusers. _Arch Gen Psychiatry_ 46: 122–126 Article CAS PubMed Google Scholar * Haertzen CA, Kocher TR, Miyasato K . (1983):
Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers,
stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. _Drug Alcohol Depend_ 11: 147–165 Article CAS PubMed Google Scholar * Haney M, Castanon N, Cador M, Le Moal M, Mormede
P . (1994): Cocaine sensitivity in Roman High and Low Avoidance rats is modulated by sex and gonadal hormone status. _Brain Res_ 645: 179–185 Article CAS PubMed Google Scholar * Hooks
MS, Jones GH, Smith AD, Neill DB, Justice JB Jr . (1991): Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. _Synapse_ 9: 121–128 Article CAS
PubMed Google Scholar * Hruska RE . (1986): Elevation of striatal dopamine receptors by estrogen: dose and time studies. _J Neurochem_ 47: 1908–1915 Article CAS PubMed Google Scholar *
Hruska RE, Nowak MW . (1988): Estrogen treatment increases the density of D1 dopamine receptors in the rat striatum. _Brain Res_ 442: 349–350 Article CAS PubMed Google Scholar * Kelly
PH . (1977): Drug-induced motor behaviour. In Iverson LL, Iversen SD, Snyder SH (eds), _Handbook in Psychopharmacology_, Eighth Edition. New York, Plenum Press, pp 295–332 Google Scholar *
Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ . (1993): Gender differences in cocaine use and treatment response. _J Subst Abuse Treat_ 10: 63–66 Article CAS PubMed Google Scholar *
Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH . (1996): Gender differences in response to intranasal cocaine administration to humans. _Biol
Psych_ 39: 147–148 Article CAS Google Scholar * Levesque D, Di Paolo T . (1988): Rapid conversion of high into low striatal D2-dopamine receptor agonist binding states after an acute
physiological dose of 17 beta-estradiol. _Neurosci Lett_ 88: 113–118 Article CAS PubMed Google Scholar * Long J, Evans HM . (1922): _The Oestrous Cycle in the Rat and Its Associated
Phenomena._ Berkeley, CA, University of California Press Google Scholar * Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH . (1996): Sex differences in
plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. _Psychopharmacology_ 125: 346–354 Article CAS PubMed Google Scholar * McDermott JL .
(1993): Effects of estrogen upon dopamine release from the corpus striatum of young and aged female rats. _Brain Res_ 606: 118–125 Article CAS PubMed Google Scholar * Mendelson JH, Weiss
R, Griffin M, Mirin SM, Teoh SK, Mello NK, Lex BW . (1991): Some special considerations for treatment of drug abuse and dependence in women. _NIDA Res Monogr_ 106: 313–327 CAS PubMed
Google Scholar * Menniti FS, Baum MJ . (1981): Differential effects of estrogen and androgen on locomotor activity induced in castrated male rats by amphetamine, a novel environment, or
apomorphine. _Brain Res_ 216: 89–107 Article CAS PubMed Google Scholar * Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW . (1991): Estradiol enhances behavioral sensitization to
cocaine and amphetamine-stimulated striatal [3H]-dopamine release. _Brain Res_ 566: 255–264 Article CAS PubMed Google Scholar * Quinones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ
. (1999): Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. _Behav Brain Res_ 101: 15–20 Article CAS PubMed Google Scholar * Randrup A,
Munkvad I . (1974): Pharmacology and physiology of stereotyped behavior. _J Psychiatr Res_ 11: 1–10 Article CAS PubMed Google Scholar * Rivest R, Falardeau P, Di Paolo T . (1995): Brain
dopamine transporter: gender differences and effect of chronic haloperidol. _Brain Res_ 692: 269–272 Article CAS PubMed Google Scholar * Robbins SJ, Ehrman RN, Childress AR, O'Brien
CP . (1999): Comparing levels of cocaine cue reactivity in male and female outpatients. _Drug Alcohol Depend_ 53: 223–230 Article CAS PubMed Google Scholar * Robinson TE . (1988):
Stimulant drugs and stress: factors influencing individual differences in the susceptibility to sensitization. In Kalivas PW, Barnes C (eds), _Sensitization in the Nervous System._ Caldwell,
NJ, Telford Press, pp 145–173 Google Scholar * Robinson TE, Becker JB, Ramirez VD . (1980): Sex differences in amphetamine-elicited rotational behavior and the lateralization of striatal
dopamine in rats. _Brain Res Bull_ 5: 539–545 Article CAS PubMed Google Scholar * Robinson TE, Camp DM, Becker JB . (1981): Gonadectomy attenuates turning behavior produced by electrical
stimulation of the nigrostriatal dopamine system in female but not male rats. _Neurosci Lett_ 23: 203–208 Article CAS PubMed Google Scholar * Robinson TE, Camp DM, Jacknow DS, Becker JB
. (1982): Sex differences and estrous cycle dependent variation in rotational behavior elicited by electrical stimulation of the mesostriatal dopamine system. _Behav Brain Res_ 6: 273–287
Article CAS PubMed Google Scholar * Savageau MM, Beatty WW . (1981): Gonadectomy and sex differences in the behavioral responses to amphetamine and apomorphine of rats. _Pharmacol
Biochem Behav_ 14: 17–21 Article CAS PubMed Google Scholar * Schneider BF, Norton S . (1979): Circadian and sex differences in hyperactivity produced by amphetamine in rats. _Physiol
Behav_ 22: 47–51 Article CAS PubMed Google Scholar * Scouten CW, Grotelueschen LK, Beatty WW . (1975): Androgens and the organization of sex differences in active avoidance behavior in
the rat. _J Comp Physiol Psychol_ 88: 264–270 Article CAS PubMed Google Scholar * Sershen H, Hashim A, Lajtha A . (1998): Gender differences in kappa-opioid modulation of cocaine-induced
behavior and NMDA-evoked dopamine release. _Brain Res_ 801: 67–71 Article CAS PubMed Google Scholar * Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U . (1987): A direct comparison of
amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. _Brain Res_ 401: 322–330 Article CAS PubMed Google Scholar * Sircar R, Kim D .
(1999): Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. _J Pharmacol Exp Ther_ 289: 54–65 CAS PubMed
Google Scholar * Stevens R, Goldstein R . (1983): Organisational effects of neonatal and pubertal testosterone on sexually differentiated behaviours in the open-field and head-dip
apparatus. _Q J Exp Psychol_ 35 (1): 81–92 Article Google Scholar * van Haaren F, Meyer ME . (1991): Sex differences in locomotor activity after acute and chronic cocaine administration.
_Pharmacol Biochem Behav_ 39: 923–927 Article CAS PubMed Google Scholar * van Hartesveldt C, Joyce JN . (1986): Effects of estrogen on the basal ganglia. _Neurosci Biobehav Rev_ 10: 1–14
Article CAS PubMed Google Scholar * van Luijtelaar ELJM, Dirksen R, Vree TB, van Haaren F . (1996): Effects of acute and chronic cocaine administration on EEG and behaviour in intact
and castrated male and intact and ovariectomized female rats. _Brain Res Bull_ 40: 43–50 Article CAS PubMed Google Scholar * Walker QD, Kuhn CM . (1997): In vivo dopamine release is
greater in female than male rats using fast-scan cyclic voltammetry. _Soc Neurosci Abstr_ 23: 693 Google Scholar * Walker QD, Lewis MH, Crofton KM, Mailman RB . (1990): Triadimefon, a
triazole fungicide, induces stereotyped behavior and alters monoamine metabolism in rats. _Toxicol Appl Pharmacol_ 102: 474–485 Article CAS PubMed Google Scholar * Walker QD, Rooney MB,
Wightman RM, Kuhn CM . (2000): Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. _Neuroscience_ 95: 1061–1070 Article CAS
PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by grant DA09079 to CMK. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pharmacology, Duke
University Medical Center, Durham, NC, USA Q David Walker Ph.D, Jose Cabassa BS, Karly A Kaplan BS, Shih-Tzung Li BS, Jennifer Haroon BS, Hilmar A Spohr MS & Cynthia M Kuhn Ph.D Authors
* Q David Walker Ph.D View author publications You can also search for this author inPubMed Google Scholar * Jose Cabassa BS View author publications You can also search for this author
inPubMed Google Scholar * Karly A Kaplan BS View author publications You can also search for this author inPubMed Google Scholar * Shih-Tzung Li BS View author publications You can also
search for this author inPubMed Google Scholar * Jennifer Haroon BS View author publications You can also search for this author inPubMed Google Scholar * Hilmar A Spohr MS View author
publications You can also search for this author inPubMed Google Scholar * Cynthia M Kuhn Ph.D View author publications You can also search for this author inPubMed Google Scholar RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Walker, Q., Cabassa, J., Kaplan, K. _et al._ Sex Differences in Cocaine-Stimulated Motor Behavior: Disparate
Effects of Gonadectomy. _Neuropsychopharmacol_ 25, 118–130 (2001). https://doi.org/10.1016/S0893-133X(00)00248-7 Download citation * Issue Date: 01 July 2001 * DOI:
https://doi.org/10.1016/S0893-133X(00)00248-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Locomotion * Rearing * Stereotypy * Gonadal hormones *
Psychostimulant