- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Dysfunction of monoamine neurotransmission seems to contribute to such pathopsychological states as depression, schizophrenia, and drug abuse. The present study examined the effects
of the selective serotonin (5-hydroxytryptamine; 5-HT) reuptake inhibitor (SSRI) and antidepressant fluvoxamine on locomotor activity in rats following administration of the catecholamine
reuptake inhibitor mazindol. Mazindol (1 mg/kg) did not alter locomotor activity; whereas, fluvoxamine (20 mg/kg) given alone induced a brief period of hypomotility. Hyperactivity was
elicited in a dose-related manner when fluvoxamine (5–20 mg/kg) was combined with mazindol (1 mg/kg). The hyperactivity elicited by fluvoxamine (20 mg/kg) plus mazindol (1 mg/kg) was
significantly attenuated by the 5-HT2A receptor antagonist M100907 (2 mg/kg) and potentiated by the 5-HT2B/2C receptor antagonist SB 206553 (2 mg/kg). Neither antagonist significantly
altered basal activity. The hyperactivity evoked by the combination of fluvoxamine and mazindol seems to be mediated in part by 5-HT2A receptors; whereas, 5-HT2B/2C receptors may serve to
limit this effect. Thus, the balance of activation between 5-HT2A and 5-HT2B/2C receptors seems to contribute to the expression of locomotor hyperactivity evoked via combination of a 5-HT
and a catecholamine reuptake inhibitor. A disruption in this balance may contribute to the expression of affective disorders, schizophrenia, and drug abuse. SIMILAR CONTENT BEING VIEWED BY
OTHERS SEROTONIN-2B RECEPTOR ANTAGONISM INCREASES THE ACTIVITY OF DOPAMINE AND GLUTAMATE NEURONS IN THE PRESENCE OF SELECTIVE SEROTONIN REUPTAKE INHIBITION Article 30 May 2020 AMPA RECEPTORS
MODULATE ENHANCED DOPAMINE NEURONAL ACTIVITY INDUCED BY THE COMBINED ADMINISTRATION OF VENLAFAXINE AND BREXPIPRAZOLE Article Open access 15 August 2024 EFFECTS OF ACUTE AND CHRONIC
ADMINISTRATION OF TRACE AMINE-ASSOCIATED RECEPTOR 1 (TAAR1) LIGANDS ON IN VIVO EXCITABILITY OF CENTRAL MONOAMINE-SECRETING NEURONS IN RATS Article Open access 01 September 2022 MAIN Several
lines of evidence suggest that dopamine (DA) and serotonin (5-hydroxy tryptamine; 5-HT) interact in the central nervous system and that dysfunction of these neurotransmitters may contribute
to such pathopsychological states as depression, schizophrenia, and drug abuse (Kahn and Davidson 1993; Kosten et al. 1998). The 5-HT-containing cell bodies of the dorsal raphe nucleus
project to DA cell bodies of the ventral tegmental area (VTA) and substantia nigra (SN), and to their terminal fields in the prefrontal cortex (PFC), nucleus accumbens (NAc), and striatum
(Hervé et al. 1987; Steinbush et al. 1981; Van der Kooy and Hattori 1980). The precise nature of the interaction between 5-HT and DA has been difficult to elucidate, with both inhibitory and
excitatory roles for 5-HT identified with respect to the neural activity of DA neurons and the release of DA. For example, electrophysiological studies in vivo suggest an inhibitory
influence of 5-HT on DA cell bodies of the VTA and SN (Prisco et al. 1994; Kelland et al. 1990), and 5-HT inhibits DA release from striatal slices (Ennis et al. 1981; Westfall and Tittermary
1982). On the other hand, in vivo microdialysis studies consistently demonstrate that local infusion of 5-HT increases DA efflux in the PFC, NAc, and striatum (Benloucif et al. 1993; Gobert
and Millan 1999; Iyer and Bradberry 1996; Parsons and Justice 1993), possibly through stimulation of one or more of the 14 5-HT receptor subtypes characterized to date (Barnes and Sharp
1999). The behavioral importance of 5-HT and DA interactions has been difficult to define. Much of this research has focused on modifications of behaviors evoked by enhanced DA
neurotransmission consequent to psychostimulant administration. In early studies, reductions and enhancements of the locomotor stimulatory effects of cocaine (Scheel-Kruger et al. 1976) and
amphetamine (Geyer et al. 1976) were reported following increased 5-HT synthesis or depletion of 5-HT, respectively. These and similar findings generated the hypothesis that 5-HT plays an
inhibitory role in DA-mediated behavior. On the other hand, systemic administration of selective serotonin reuptake inhibitors (SSRIs) potentiated hyperactivity induced by amphetamine or
cocaine in rats (Herges and Taylor 1998; Sills et al. 1999a, 1999b), but not in mice (Arnt et al. 1984; Maj et al. 1984; Reith et al. 1991). The ability of SSRIs to increase 5-HT efflux is
well-documented (e.g., Guan and McBride 1988; Li et al. 1996), thus, these data imply a more sophisticated role for 5-HT in the control of DA-mediated behaviors than simple inhibition. The
mechanisms that underlie the potentiation of DA-mediated hyperactivity induced by SSRIs have been little studied. A possible pharmacokinetic interaction at the level of metabolic enzymes has
been suggested to contribute to the potentiation of amphetamine-induced hyperactivity by SSRIs (Sills et al. 1999a, 1999b). However, a role for specific 5-HT receptors is suggested by the
observation that the facilitation of cocaine-induced hyperactivity by fluoxetine was attenuated by the 5-HT1A receptor antagonist WAY 100635 and the nonselective 5-HT2 receptor antagonist
ketanserin (Herges and Taylor 1998). The goal of the present study was to analyze further the 5-HT2 receptor subtypes involved in SSRI-evoked potentiation of DA-elicited behaviors. To
conduct these studies, we chose to utilize the catecholamine reuptake inhibitor mazindol, rather than the catecholamine releaser amphetamine (Heikkila et al. 1975) or cocaine that blocks the
transporters for DA (Ki = 277 nM), 5-HT (Ki = 217 nM) and norepinep;hrine (NE; Ki = 144 nM) in rat brain synaptosomes (Koe 1976; Hyttel 1982). Mazindol binds with greater affinity to
transporters for DA (Ki = 16 nM), 5-HT (Ki = 25 nM), and NE (Ki = 0.42 nM; Hyttel 1982) in rat brain synaptosomes, but has been characterized in vitro and in vivo as a potent inhibitor of DA
and NE reuptake with less potency for 5-HT reuptake (Heikkila et al. 1977; Sugrue et al. 1977). The behavioral effects of mazindol have been attributed to catecholamine reuptake inhibition,
especially that of DA, as DA receptor antagonists have been shown to attenuate both mazindol-induced anorexia and locomotor hyperactivity (Gevaerd and Takahashi 1999; Kruk and Zarrindast
1976). The present study was designed to assess the hyperactivity evoked by combinations of the SSRI fluvoxamine with mazindol and the involvement of 5-HT2 receptors in the interactive
behavioral effects of these two drugs. The 5-HT2A and 5-HT2C receptors were hypothesized to be particularly important because of the moderate to dense localization of both transcript and
protein for these receptors in SN and VTA as well as DA terminal regions of rat forebrain (Abramowski et al. 1995; Lopez-Gimenez et al. 1997; Pompeiano et al. 1994). Antagonism of 5-HT2A (De
Deurwaerdere and Spampinato 1999; Lucas and Spampinato 2000) and 5-HT2C receptors (De Deurwaerdere and Spampinato 1999; Di Giovanni et al. 1999; Di Matteo et al. 1998; Lucas and Spampinato
2000) has been shown to influence DA function in areas of brain (e.g., NAc and striatum) thought to be important in motor activation, motivation, and reward. In the present study, locomotor
activity was assessed following administration of the SSRI fluvoxamine in combination with a low dose of mazindol in rats. The selective 5-HT2A receptor antagonist M100907 (Sorensen et al.
1993) and the 5-HT2B/2C receptor antagonist SB 206553 (Kennett et al. 1996) were employed to analyze the roles of these receptors in hyperactivity evoked by fluvoxamine plus mazindol. The
doses of these 5-HT2 receptor antagonists were chosen based on their documented efficacy following systemic administration (McCreary and Cunningham 1999; McMahon and Cunningham submitted;
Moser et al. 1996). METHODS ANIMALS The subjects were 36 experimentally naive male Sprague–Dawley rats (Harlan, Houston, TX) weighing between 300–350 g at the beginning of the study. The
rats were housed in pairs in a colony room that was maintained at a constant temperature (21–23°C) and humidity (40–50%); lighting was maintained on a 12-h light–dark cycle (07:00–19:00 h).
Each rat was provided with continuous access to tap water and rodent chow throughout the experiment except during experimental sessions. APPARATUS Locomotor activity was monitored and
quantified using an open field activity system (San Diego Instruments, San Diego, CA). Each clear _Plexiglas_ chamber (40 cm × 40 cm × 40 cm) was housed within sound-attenuating enclosures
and was surrounded with a 4 × 4 photobeam matrix located 4 cm from the floor surface. Interruptions of the photobeams resulted in counts of activity in the peripheral and central fields of
the chamber. Activity recorded in the inner 16 × 16 cm of the open field was counted as central activity, while the field bounded by the outer 16-cm band registered peripheral activity.
Separate counts of peripheral and central activity were made by the control software (Photobeam Activity Software, San Diego Instruments) and stored for subsequent statistical evaluation.
Peripheral and central activity counts were summed to provide a single measure of total horizontal activity. Video cameras positioned above the chambers permitted continuous observation of
behavior without disruption. BEHAVIORAL PROCEDURES All rats were maintained in the colony room for a minimum of 1 week before behavioral testing for acclimation to daily handling procedures.
At the time tests were to be conducted (between 09:00–12:00 h), all rats were habituated to the testing environment for 2 h per day on each of the 2 days before the start of the experiment.
On each of the test days, rats were habituated to the activity monitors for 1 h before administration of drugs. In Experiment 1, the group of rats (n = 13) received an injection of either
saline (1 ml/kg, IP) or fluvoxamine (5, 10, or 20 mg/kg, IP), followed 30 min later by an injection of either saline (1 ml/kg, IP) or mazindol (1 mg/kg, IP). In Experiment 2, the group of
rats (n = 10) received an injection of either vehicle (1 ml/kg of 1% Tween 80, IP) or M100907 (2 mg/kg, IP), followed 10 min later by an injection of either saline (1 ml/kg, IP) or
fluvoxamine (20 mg/kg, IP), followed 30 min later by an injection of either saline (1 ml/kg, IP) or mazindol (1 mg/kg, IP). In Experiment 3, the group of rats (n = 13) received an injection
of either vehicle (1 ml/kg of 45% β-cyclodextrin, IP) or SB 206553 (2 mg/kg, IP), followed 10 min later by an injection of either saline (1 ml/kg, IP) or fluvoxamine (20 mg/kg, IP), followed
30 min later by an injection of either saline (1 ml/kg, IP) or mazindol (1 mg/kg, IP). Rats within a given group received each of the experimental treatments assigned to that group for a
total of eight tests that were randomized using a Latin square design. Doses of M100907 (McMahon and Cunningham in press) and SB 206553 (McCreary and Cunningham 1999) were chosen based upon
our previous experience with these drugs. Measurement of locomotor activity counts began immediately following the mazindol or saline injection and was divided into 5-min bins for a total of
90 min for each of the groups. For each rat, the order of drug tests for the antagonists and fluvoxamine were counterbalanced, and the mazindol injections were given every other test. Test
sessions were conducted every 2 to 3 days. Thus, mazindol injections occurred no more than once per week. DATA ANALYSIS Data were analyzed as horizontal (peripheral plus central) activity
counts totaled for the 90-min test session or in 18 separate 5-min time bins following IP injection of mazindol or its saline control. Because group comparisons were specifically defined
before the start of the experiment, these planned comparisons were conducted in lieu of an over-all F test in a multifactorial analysis of variance (ANOVA); this statistical analysis has
been supported in a number of statistical texts (e.g., Keppel 1973). Thus, each experiment was subjected to a one-way ANOVA for repeated measures with levels of the treatment factor
corresponding to the eight drug combinations administered to that group. Planned, pair-wise comparisons of the treatment means were made with Student-Newman-Keuls procedure (SAS for Windows,
Version 6.12). Treatment × time interactions were analyzed using a two-way ANOVA for repeated measures. All statistical analyses were conducted with an experiment-wise error rate of α =
0.05. DRUGS Doses of all drugs refer to the weight of the salt. Cocaine hydrochloride (NIDA) and fluvoxamine maleate (Solvay, Marietta, GA) were prepared in 0.9% NaCl. Mazindol (Sandoz,
Hanover, NJ) was prepared in 0.9% NaCl with mild acidification. SB 206553 [5-methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole); SmithKline Beecham, Frythe, Welwyn, UK]
was prepared in 45% 2-hydroxypropyl-β-cyclodextrin (Sigma/RBI, Natick, MA). M100907 [R-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol; Hoechst Marion Roussel,
Cincinnati, OH] was prepared in a solution of 1% Tween 80 (Sigma, St. Louis, MO) in sterile distilled water. All drugs were injected IP in a volume of 1 ml/kg. RESULTS EFFECTS OF THE SSRI
FLUVOXAMINE ALONE OR IN COMBINATION WITH THE CATECHOLAMINE UPTAKE INHIBITOR MAZINDOL In Experiment 1, the effects of saline or fluvoxamine (5, 10 or 20 mg/kg) in combination with saline or
mazindol (1 mg/kg) were assessed. A dose of 1 mg/kg of mazindol was chosen for these analyses, because this dose was subthreshold for elicitation of hyperactivity, unlike 2 and 5 mg/kg,
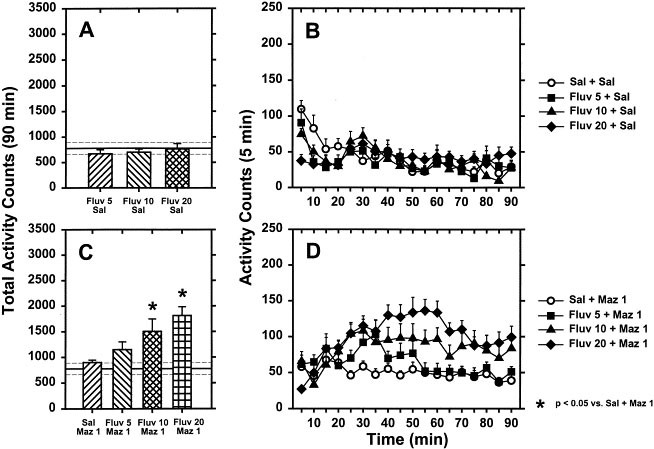
which evoked significant hyperactivity (data not shown). A main effect of treatment on total horizontal activity counts was observed [F(7,96) = 9.69, p < .001]. For visual simplicity,
these data are graphed separately in Figure 1 top (A and B) and bottom (C and D). No significant differences in total horizontal activity were observed after 5, 10 or 20 mg/kg of fluvoxamine
(Figure 1 A and B ) or mazindol (1mg/kg; Figure 1C and D) compared to saline controls (_p_> .05). In contrast, significant increases in total horizontal activity were observed after
combination of fluvoxamine (10 or 20 mg/kg) and mazindol (1 mg/kg; Figure 1 C and D ) compared to mazindol alone (_p_ < .05). A significant treatment × time interaction was observed for
horizontal activity [F(126,1386) = 3.41, p < .001]. Fluvoxamine alone decreased horizontal activity at the beginning of the test session (<30 min; Figure 1B). Fluvoxamine at all doses
in combination with mazindol produced significant hyperactivity compared to injection of mazindol alone beginning ∼25 min after mazindol injection. The hyperactivity induced by mazindol in
combination with 10 or 20 mg/kg of fluvoxamine was of longer duration than that observed following 5 mg/kg of fluvoxamine plus mazindol (Figure 1D). EFFECTS OF THE 5-HT2A RECEPTOR ANTAGONIST
M100907 ON LOCOMOTOR ACTIVITY EVOKED BY MAZINDOL ALONE OR IN COMBINATION WITH FLUVOXAMINE In Experiment 2, the effects of vehicle or M100907 (2 mg/kg) on horizontal activity evoked by
saline or fluvoxamine (20 mg/kg) in combination with saline or mazindol (1 mg/kg) were assessed. A main effect of treatment on total horizontal activity counts was observed [F(7,72) = 10.48,
p < .001]. For visual simplicity, these data are graphed separately in Figure 2 top (A and B) and bottom (C and D). Basal horizontal activity observed in vehicle-saline-saline controls
was not significantly altered by fluvoxamine, M100907, or co-administration of M100907 plus fluvoxamine (_p_> .05; Figure 2 A and B ). As noted in Experiment 1, total horizontal activity
was significantly increased by the combination of fluvoxamine and mazindol compared to that observed after mazindol alone (_p_ < .05; Figure 2 C and D ). In contrast, horizontal activity
after M100907 in combination with fluvoxamine and mazindol was not significantly different from basal activity or that observed after mazindol alone (_p_> .05; Figure 2C). M100907 blocked
the hyperactivity seen with fluvoxamine plus mazindol for the duration of the session (Figure 2D). Horizontal activity after M100907 in combination with mazindol alone was significantly
different from that observed after mazindol alone (_p_ < .05) but not from vehicle-saline-saline controls (_p_> .05; Fig. 2C). A significant treatment × time interaction was observed
for horizontal activity in 5-min time bins [F(119,1071) = 2.98, p < .001]. As noted in Experiment 1, fluvoxamine depressed activity during the first 30 min of the session (Figure 2B).
EFFECTS OF THE 5-HT2B/2C RECEPTOR ANTAGONIST SB 206553 ON LOCOMOTOR ACTIVITY EVOKED BY MAZINDOL ALONE OR IN COMBINATION WITH FLUVOXAMINE In Experiment 3, the effects of vehicle or SB 206553
(2 mg/kg) on horizontal activity evoked by saline or fluvoxamine (20 mg/kg) in combination with saline or mazindol (1 mg/kg) were assessed. A main effect of treatment on total horizontal
activity counts was observed [F(7,96) = 16.93, p < .001]. For visual simplicity, these data are graphed separately in Figure 3 top (A and B) and bottom (C and D). In this experiment, a
significant decrease in activity was observed after fluvoxamine compared to vehicle-saline-saline controls (_p_ < .05; Figure 3 A and B ). In contrast, horizontal activity after SB 206553
in combination with saline or fluvoxamine was not significantly different from that observed in vehicle-saline-saline controls (_p_> .05; Figure 3 A and B ). As noted in Experiments 1
and 2, total horizontal activity was significantly increased by the combination of fluvoxamine and mazindol compared to that observed after mazindol alone (_p_ < .05; Figure 3 C and D ).
Pretreatment with SB 206553 (2 mg/kg) significantly potentiated hyperactivity evoked by injections of fluvoxamine plus mazindol (_p_ < .05; Fig. 3C and D). A significant treatment × time
interaction was observed for horizontal activity in 5-min time bins [F(119,1428) = 5.33, p < .001]. Fluvoxamine-induced hypoactivity was evident during the first 30 min of the test
session and was reversed by SB 206553 (Figure 3B). In addition, the peak and duration of hyperactivity observed after the fluvoxamine-mazindol combination was extended by pretreatment with
SB 206553 (Figure 3D). DISCUSSION Based on previous evidence that 5-HT can influence DA function (see Introduction), the present study examined locomotor activity in rats after combination
of the SSRI fluvoxamine and the catecholamine reuptake inhibitor mazindol. Mazindol alone at doses of 2 or 5 mg/kg induced a dose-related increase in locomotor activity that was
characterized by a peak hyperactivity at 10 to 15 min postinjection and a duration of at least 90 min (maximum duration of the current test sessions; data not shown). Hyperactivity evoked by
mazindol has been ascribed to inhibition of DA reuptake and is blocked by DA D1- and D2-like antagonists (Gevaerd and Takahashi 1999; Ross 1979). On the other hand, fluvoxamine tended to
depress activity, particularly at the highest dose tested (20 mg/kg). This hypoactivity occurred 30 to 60 min after fluvoxamine injection and is most probably related to the flat body
posture observed at that time point (data not shown), in the absence of other signs of the 5-HT syndrome (Grahame-Smith 1971). However, this hypomotility was short-lived, and the total
horizontal activity for the duration of the session (90 min) was not significantly affected in two out of the three tests with 20 mg/kg of fluvoxamine. Minimal effects of fluvoxamine on
locomotor activity have also been reported in mice (Maj et al. 1983; Reith et al. 1991). Because fluvoxamine is an SSRI that possesses no appreciable affinity for NE or DA transporters or
monoamine receptors (Wong et al. 1983; Hyttel 1994), reductions in locomotor activity evoked by fluvoxamine may be caused by elevated levels of endogenous 5-HT acting at specific 5-HT
receptors (Benloucif et al. 1993; Gobert and Millan 1999; Iyer and Bradberry 1996; Parsons and Justice 1993). The 5-HT2B/2C receptor antagonist SB 206553, but not the 5-HT2A receptor
antagonist M100907, reversed the initial suppression of activity evoked by fluvoxamine. Hypoactivity resulting from activation of 5-HT2C receptors has been well characterized by others
(e.g., Curzon and Kennett 1990) and the present results suggest that indirect activation of 5-HT2C receptors following reuptake inhibition may account for the transient induction of
hypoactivity produced by fluvoxamine. Fluvoxamine (5–20 mg/kg) dose-dependently evoked hyperactivity when given in combination with a dose of mazindol (1 mg/kg). This dose of mazindol was
subthreshold for the elicitation of observable locomotor activation, but is a dose that has been reported to increase extracellular DA concentrations in rat striatum (Ng et al. 1992). These
doses of fluvoxamine were reported to increase extracellular levels of 5-HT in frontal cortex without altering extracellular levels of DA or NE (Jordan et al. 1994). Based on these
neurochemical data, one possible mechanism by which hyperactivity is elicited by fluvoxamine in combination with mazindol is via a 5-HT receptor-mediated enhancement of catecholaminergic
neurotransmission. Such a mechanism would involve inhibition of 5-HT reuptake by fluvoxamine and indirect stimulation of specific 5-HT receptor subtypes. The selective 5-HT2A receptor
antagonist M100907, at a dose previously shown to block the in vivo effects associated with 5-HT2A receptor stimulation (Kehne et al. 1996b; Sorensen et al. 1993), reversed the hyperactivity
induced by co-administration of fluvoxamine plus mazindol. M100907 is one of the few ligands available that has been shown to cross the blood–brain barrier and to discriminate between
5-HT2A and 5-HT2C receptors. In fact, M100907 possesses a 100-fold greater affinity for 5-HT2A receptors (Ki = 0.85 nM) over 5-HT2C (Ki = 88 nM) and α1-adrenergic receptors (Ki = 128 nM),
and negligible affinity for most other receptors, including DA D1- and D2-like receptors (>500 nM; Kehne et al. 1996a). The selectivity of M100907 is further suggested by the observation
that doses of M100907 up to 30 times higher than those used here did not antagonize the behavioral effects of 5-HT2C, D2-like and α1-adrenergic agonists (Dekeyne et al. 1999; Kehne et al.
1996a). Thus, the efficacy of M100907 to block hyperactivity is most likely attributable to a selective antagonism of 5-HT2A receptors in vivo. The level of tonic regulation of DA
neurotransmission provided by 5-HT2A receptors is somewhat controversial. Systemic injection of 5-HT2A receptor antagonists did not potently alter basal behavior (present results; Kehne et
al. 1996a, 1996b; McMahon and Cunningham in press; Sorensen et al. 1993; but see Gleason and Shannon 1998), basal cellular activity of DA somata (Sorensen et al. 1993) or striatal (Schmidt
et al. 1992), accumbal (De Deurwaerdere and Spampinato 1999) or cortical DA efflux (Gobert and Millan 1999), suggesting that 5-HT2A receptors provide little tonic control over DA function.
The failure of the 5-HT2A/2B/2C receptor agonist 1-(2,5-dimethoxy-4-iodo)-2-aminopropane (DOI) to evoke striatal DA efflux in the presence of the 5-HT2B/2C receptor antagonist SB 206553
suggests further that selective 5-HT2A receptor activation is not sufficient to provoke DA efflux in this DA terminal field (Lucas and Spampinato, 2000). However, under conditions of DA
stimulation, 5-HT2A receptors do seem to modulate DA outflow positively. For example, antagonism of 5-HT2A receptors has been shown to attenuate striatal DA efflux stimulated by systemic
administration of (±)-3,4-methylenedioxymethamphetamine (MDMA; Schmidt et al. 1992), blockade of D2-like autoreceptors with haloperidol (Lucas and Spampinato 2000), and electrical
stimulation of the dorsal raphe nucleus (De Deurwaerdere and Spampinato 1999). Systemic administration of M100907 also attenuated the suppression of DA cell firing evoked by amphetamine
(Sorensen et al. 1993) and locomotor hyperactivity induced by amphetamine (Moser et al. 1996; Sorensen et al. 1993), cocaine (McMahon and Cunningham in press) or MDMA (Kehne et al. 1996b).
Thus, the combination of fluvoxamine and mazindol mimics the activation of DA function under which 5-HT2A receptors become functional, conditions under which M100907 is an effective
antagonist of the resulting hyperactivity. The mechanisms, triggers, and sites of action for 5-HT2A receptors to control stimulated DA function have not yet been thoroughly clarified,
although the mechanism may involve blockade of 5-HT2A receptors that putatively control DA synthesis under conditions of stimulated DA neurotransmission (Lucas and Spampinato 2000; Schmidt
et al. 1992). Despite the evidence to suggest that 5-HT2A receptors exercise little tonic control over DA function under normal conditions (above), basal 5-HT concentrations may provide
sufficient tone on 5-HT2A receptors such that, under conditions of stimulated DA neurotransmission, antagonism of 5-HT2A receptors triggers functional mechanisms that compensate for the
overactivation of DA neurons. Such a mechanism might help to explain observations that M100907 can block the in vivo consequences of drugs thought to result predominantly in enhanced DA
efflux, such as amphetamine (Moser et al. 1996; Sorensen et al. 1993), the DA reuptake inhibitor GBR 12909 (Carlsson 1995) and mazindol given alone (present results; Figure 2). On the other
hand, further increases in interstitial levels of 5-HT, such as following cocaine (McMahon and Cunningham in press), the 5-HT- and DA-releaser MDMA (Kehne et al. 1996b; Schmidt et al. 1992)
or a combination of fluvoxamine plus mazindol (present results), may be required to uncover the control of DA function by 5-HT2A receptors. In this case, one must postulate that drugs such
as amphetamine, GBR 12909 and mazindol might alter interstitial levels of 5-HT, possibly below the limits of detectability for microdialysis experiments, that could contribute to the ability
of 5-HT2A receptors to control DA function. To complicate matters further, in addition to 5-HT-evoked increases in DA efflux (Benloucif et al. 1993; Gobert and Millan 1999; Iyer and
Bradberry 1996; Parsons and Justice 1993), DA has also been shown to increase 5-HT release (Matsumoto et al. 1996), and these effects seem to be mediated, at least in part, by stimulation of
specific 5-HT and DA receptors, respectively. In contrast to the 5-HT2A receptor antagonist M100907, pretreatment with the 5-HT2B/2C receptor antagonist SB 206553 (2 mg/kg) potentiated the
hyperactivity elicited by fluvoxamine plus mazindol. Doses of SB 206553 in this range have previously been shown to effectively inhibit hypomotility induced by the 5-HT2 receptor agonist
m-chlorophenylpiperazine (Heisler and Tecott 2000) and the stimulus effects of the 5-HT2C receptor agonist RO 60-0175 (Dekeyne et al. 1999), with little evidence of behavioral disruption
when given alone (present results; Dekeyne et al. 1999; Kennett et al. 1996). The potentiation produced by SB 206553 may have involved the removal of a tonic, inhibitory influence of 5-HT2C
receptors on DA neurotransmission. In support of this hypothesis, SB 206553 was found to increase the firing rate of DA neurons in the VTA (Di Giovanni et al. 1999) and striatal DA efflux
(De Deurwaerdere and Spampinato 1999; Di Matteo et al. 1998). Activation of 5-HT2C receptors consequent to 5-HT reuptake inhibition induced by fluvoxamine might have reduced DA function
stimulated by mazindol, thereby self-limiting the locomotor activation produced by the combination of fluvoxamine plus mazindol. As noted above, hypoactivity resulting from activation of
5-HT2C receptors has been well characterized by others (Curzon and Kennett 1990). The present study suggests that the locomotor response to increased synaptic 5-HT in the face of modest
catecholamine efflux depends upon the balance of 5-HT2A and 5-HT2C receptor activation. Thus, 5-HT2A and 5-HT2C receptors may serve opposing roles in the control of behaviors associated with
increased catecholamine neurotransmission. Although we have focused the discussion on 5-HT-DA interactions, 5-HT interactions with NE (e.g., Saito et al. 1996) may be equally important,
particularly given the affinity of mazindol for NE transporters (Hyttel 1982), and should not be overlooked. A second mechanism that may account in part for the hyperactivity induced by the
combination of fluvoxamine and mazindol involves potential pharmacokinetic interactions between these monoamine reuptake inhibitors. If acute administration of fluvoxamine impedes the
metabolism of mazindol via inhibition of metabolic enzymes, increased brain concentrations of mazindol could contribute to the observed hyperactivity. A similar mechanism was proposed for
the facilitation of amphetamine-induced hyperactivity and DA efflux in NAc by systemic fluoxetine; in that study, increased amphetamine levels were observed in the NAc of fluoxetine-treated
rats (Sills et al. 1999a). Fluvoxamine has been shown to inhibit cytochrome P450 isozymes that catalyze the oxidative metabolism of certain drugs and could, therefore, increase brain levels
of drugs metabolized by these isozymes (Hiemke and Hartter 2000). The ability of cytochrome P450 isozymes to contribute to the transformation of mazindol into imidazole-3-one, its primary
metabolite in the rat, is currently unknown (Dugger et al. 1979). In vivo microdialysis is one strategy that could be used to determine the extent to which the observed synergism between
fluoxetine and mazindol might be related to fluvoxamine-evoked increases in brain concentrations of mazindol (Sills et al. 1999a). However, we have recently shown that microinjections of
fluvoxamine into the shell of the NAc result in an immediate enhancement of hyperactivity evoked by systemic cocaine (McMahon et al. 2000), a finding difficult to reconcile with a mechanism
dependent upon inhibition of metabolism. Future studies of this nature as well as the establishment of a full dose response curve for mazindol in the presence of multiple doses of
fluvoxamine will help to clarify the contribution of metabolic processes to this effect. In conclusion, these results suggest that endogenous 5-HT regulates behavioral states ensuing from
stimulated catecholamine neurotransmission, possibly by exerting excitatory and inhibitory tone on behavior through actions at 5-HT2A and 5-HT2C receptors, respectively. The present study
further suggests that the balance of activation between 5-HT2A and 5-HT2C receptors may contribute to pathopsychological states associated with dysfunction of monoamine neurotransmission.
Thus, 5-HT2A and 5-HT2C receptors might prove to be important targets for the successful development of pharmacotherapies for affective disorders, schizophrenia, and drug abuse.Heisler
Tecott 2000 REFERENCES * Abramowski D, Rigo M, Duc D, Hoyer D, Staufenbiel M . (1995): Localization of the 5-hydroxytryptamine2C receptor protein in human and rat brain using specific
antisera. _Neuropharmacology_ 34: 1635–1645 Article CAS Google Scholar * Arnt J, Hyttel J, Overo KF . (1984): Prolonged treatment with the specific 5-HT-uptake inhibitor citalopram:
Effect on dopaminergic and serotonergic functions. _J Pharm Pharmacol_ 36: 221–230 CAS Google Scholar * Barnes NM, Sharp T . (1999): A review of central 5-HT receptors and their function.
_Neuropharmacology_ 38: 1083–1152 Article CAS Google Scholar * Benloucif S, Keegan MJ, Galloway MP . (1993): Serotonin-facilitated dopamine release in vivo: Pharmacological
characterization. _J Pharmacol Exp Ther_ 265: 373–377 CAS Google Scholar * Carlsson ML . (1995): The selective 5-HT2A receptor antagonist MDL 100907 counteracts the psychomotor stimulation
ensuing manipulations with monoaminergic, glutamatergic, or muscarinic neurotransmission in the mouse—Implications for psychosis. _J Neural Transm_ 100: 225–237 Article CAS Google Scholar
* Curzon G, Kennett GA . (1990): m-CPP: A tool for studying behavioral responses associated with 5-HT(1C) receptors. _Trends Pharmacol Sci_ 11: 181–182 Article CAS Google Scholar * De
Deurwaerdere P, Spampinato U . (1999): Role of serotonin2A and serotonin2C receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus
electrical stimulation. _J Neurochem_ 73: 1033–1042 Article CAS Google Scholar * Dekeyne A, Girardon S, Millan MJ . (1999): Discriminative stimulus properties of the novel serotonin
(5-HT) 5-HT2C receptor agonist, RO 60-0175: A pharmacological analysis. _Neuropharmacology_ 38: 415–423 Article CAS Google Scholar * Di Giovanni G, De Deurwaerdere P, Di Mascio M, Di
Matteo V, Esposito E, Spampinato U . (1999): Selective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: A combined in vivo
electrophysiological and microdialysis study. _Neuroscience_ 91: 587–597 Article CAS Google Scholar * Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E . (1998): Selective blockade of
serotonin2B/2C receptors enhances dopamine release in the rat nucleus accumbens. _Neuropharmacology_ 37: 265–272 Article CAS Google Scholar * Dugger HA, Madrid VO, Talbot KC, Coombs RA,
Orwig BA . (1979): Biotransformation of mazindol. III. Comparison of metabolism in rat, dog, and man. _Drug Metab Dispos_ 7: 132–137 CAS Google Scholar * Ennis C, Kemp JD, Cox B . (1981):
Characterization of inhibitory 5-hydroxytryptamine receptors that modulate dopamine release in the striatum. _J Neuroschem_ 36: 1515–1520 Article CAS Google Scholar * Gevaerd MS,
Takahashi RN . (1999): Involvement of dopamine receptors on locomotor stimulation and sensitization elicited by the interaction of ethanol and mazindol in mice. _Pharmacol Biochem Behav_ 63:
395–399 Article CAS Google Scholar * Geyer MA, Puerto A, Menkes DB, Segal DS, Mandell AJ . (1976): Behavioral studies following lesions of the mesolimbic and mesostriatal serotonergic
pathways. _Brain Res_ 106: 257–270 Article CAS Google Scholar * Gleason SD, Shannon HE . (1998): Meta-chlorophenylpiperazine induced changes in locomotor activity are mediated by 5-HT1
and 5-HT2c receptors in mice. _Eur J Pharmacol_ 341: 135–138 Article CAS Google Scholar * Gobert A, Millan MJ . (1999): Serotonin (5-HT)2A receptor activation enhances dialysate levels of
dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely moving rats. _Neuropharmacology_ 38: 315–317 Article CAS Google Scholar * Grahame-Smith DG . (1971): Studies in
vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. _J Neurochem_ 18: 1053–1066
Article CAS Google Scholar * Guan XM, McBride WJ . (1988): Fluoxetine increases the extracellular levels of serotonin in the nucleus accumbens. _Brain Res Bull_ 21: 43–46 Article CAS
Google Scholar * Heikkila RE, Cabbat FS, Mytilineou C . (1977): Studies on the capacity of mazindol and dita to act as uptake inhibitors or releasing agents for 3H-biogenic amines in rat
brain tissue slices. _Eur J Pharmacol_ 45: 329–333 Article CAS Google Scholar * Heikkila RE, Orlansky H, Mytilienou C, Cohen G . (1975): Amphetamine: Evaluation of D- and L-isomers as
releasing agents and uptake inhibitors for 3H-dopamine and 3H-norepinephrine in slices of rat neostriatum and cerebral cortex. _J Pharmacol Exp Ther_ 194: 47–56 CAS Google Scholar *
Heisler LK, Tecott LH . (2000): A paradoxical locomotor response in serotonin 5-HT2C receptor mutant mice. _J Neurosci_ 20: RC71 Article CAS Google Scholar * Herges S, Taylor DA . (1998):
Involvement of serotonin in the modulation of cocaine-induced locomotor activity in the rat. _Pharmacol Biochem Behav_ 59: 595–611 Article CAS Google Scholar * Hervé RM, Pickel VM, Joh
TH, Beaudet A . (1987): Serotonin axon terminals in the ventral tegmental area of the rat: Fine structure and synaptic input to dopaminergic neurons. _Brain Res_ 435: 71–83 Article Google
Scholar * Hiemke C, Hartter S . (2000): Pharmacokinetics of selective reuptake inhibitors. _Pharmacol Ther_ 85: 11–28 Article CAS Google Scholar * Hyttel J . (1982): Citalopram:
Pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. _Prog Neuro-Psychopharmacol Biol Psychiat_ 6: 277–295 Article CAS Google Scholar * Hyttel J
. (1994): Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs). _Int Clin Psychopharmacol_ 9: 19–26 Article Google Scholar * Iyer RN, Bradberry CW . (1996):
Serotonin-mediated increase in prefrontal cortex dopamine release: Pharmacological characterization. _J Pharmacol Exp Ther_ 277: 40–47 CAS Google Scholar * Jordan S, Kramer GL, Zukas PK,
Moeller M, Petty F . (1994): In vivo biogenic amine efflux in medial prefrontal cortex with imipramine, fluoxetine, and fluvoxamine. _Synapse_ 18: 294–297 Article CAS Google Scholar *
Kahn RS, Davidson M . (1993): Serotonin, dopamine and their interactions in schizophrenia. _Psychopharmacology_ 112: S1–S4 Article CAS Google Scholar * Kehne JH, Baron BM, Carr AA, Chaney
SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PLM, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW and Widmaier C . (1996a): Preclinical characterization of the
potential of the putative atypical antipsychotic MDL 100907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. _J Pharmacol Exp Ther_ 277: 968–981 CAS Google Scholar *
Kehne JH, Ketteler HJ, McCloskey TC, Sullivan CK, Dudley MW, Schmidt CJ . (1996b): Effects of the selective 5-HT2A antagonist M100907 on MDMA-induced locomotor stimulation in rats.
_Neuropsychopharmacology_ 15: 116–124 Article CAS Google Scholar * Kelland MD, Freeman AS, Chiodo LA . (1990): Serotonin afferent regulation of the basic physiology and pharmacological
responsiveness of nigrostriatal dopamine neurons. _J Pharmacol Exp Ther_ 253: 803–811 CAS Google Scholar * Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, Gager T, Thomas D, Baxter GS,
Forbes IT, Ham P, Blackburn TP . (1996): In vitro and in vivo profiles of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. _Br J Pharmacol_ 117: 427–434
Article CAS Google Scholar * Keppel G . (1973): _Design and Analysis: A Researcher's Handbook._ Upper Saddle River, NJ: Prentice-Hall Google Scholar * Koe BK . (1976): Molecular
geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. _J Pharmacol Exp Ther_ 199: 649–661 CAS Google Scholar * Kosten TR, Markou
A, Koob GF . (1998): Depression and stimulant dependence: Neurobiology and pharmacotherapy. _J Nerv Ment Dis_ 186: 737–745 Article CAS Google Scholar * Kruk ZL, Zarrindast MR . (1976):
Mazindol anorexia is mediated by activation of dopaminergic systems. _Br J Pharmacol_ 58: 367–372 Article CAS Google Scholar * Li M-Y, Yan Q-S, Coffey LL, Reith MEA . (1996):
Extracellular dopamine, norepinephrine, and serotonin in the nucleus accumbens of freely moving rats during intracerebral dialysis with cocaine and other monoamine uptake blockers. _J
Neurochem_ 66: 559–568 Article CAS Google Scholar * Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT . (1997): Selective visualization of rta brain 5-HT2A receptors by autoradiography
with [3H]M100907. _Naunyn–Schmiedeberg's Arch Pharmacol_ 356: 446–454 Article CAS Google Scholar * Lucas G, Spampinato U . (2000): Role of striatal serotonin2A and serotonin2C
receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. _J Neurochem_ 74: 693–701 Article CAS Google Scholar * Maj J, Rogoz Z, Skuza G, Sowinska H . (1983):
Reserpine-induced locomotor stimulation in mice chronically treated with typical and atypical antidepressants. _Eur J Pharmacol_ 87: 469–474 Article CAS Google Scholar * Maj J, Rogoz Z,
Skuza G, Sowinska H . (1984): Repeated treatment with antidepressant drugs potentiates the locomotor response to (+)-amphetamine. _J Pharm Pharmacol_ 36: 127–130 Article CAS Google Scholar
* Matsumoto M, Yoshioka M, Togashi H, Ikeda T, Saito H . (1996): Functional regulation by dopamine receptors of serotonin release from the rat hippocampus: In vivo microdialysis study.
_Naunyn–Schmiedebergs Arch Pharmacol_ 353: 621–629 Article CAS Google Scholar * McCreary A, Cunningham KA . (1999): Effects of the 5-HT2C/2B antagonist SB 206553 on hyperactivity induced
by cocaine. _Neuropsychopharmacology_ 20: 556–564 Article CAS Google Scholar * McMahon LR, Bubar MJ, Cunningham KA . (2000): Potentiation of cocaine-induced hyperactivity by infusion of
selective serotonin reuptake inhibitors into the rat nucleus accumbens shell. _Soc Neurosci Abstr_ 25, 1824 Google Scholar * McMahon LR, Cunningham KA . (in press): Antagonism of
5-hydroxytryptamine2A receptors attenuates the behavioral effects of cocaine in rats. _J Pharmacol Exp Ther_ * Moser PC, Moran PM, Frank RA, Kehne JH . (1996): Reversal of
amphetamine-induced behaviors by M100907, a selective 5-HT2A antagonist. _Behav Brain Res_ 73: 163–167 Article CAS Google Scholar * Ng JP, Menacherry SD, Liem BJ, Anderson D, Singer M,
Justice JB . (1992): Anomalous effect of mazindol on dopamine uptake as measured by in vivo voltametry and microdialysis. _Neurosci Lett_ 134: 229–232 Article CAS Google Scholar * Parsons
LH, Justice JB . (1993): Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. _Brain Res_ 606: 195–199 Article CAS Google
Scholar * Pompeiano M, Palacios JM, Mengod G . (1994): Distribution of serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. _Mol Brain Res_ 23: 163–178
Article CAS Google Scholar * Prisco S, Pagannone S, Esposito E . (1994): Serotonin–dopamine interaction in the rat ventral tegmental area: An electrophysiological study in vivo. _J
Pharmacol Exp Ther_ 271: 83–90 CAS Google Scholar * Reith MEA, Wiener HL, Fischette CT . (1991): Sertraline and cocaine-induced locomotion in mice. _Psychopharmacology_ 103: 297–305
Article CAS Google Scholar * Ross SB . (1979): The central stimulatory action of inhibitors of the dopamine uptake. _Life Sci_ 24: 159–168 Article CAS Google Scholar * Saito H,
Matsumoto M, Togashi H, Yoshioka M . (1996): Functional interaction between serotonin and other neuronal systems: Focus on in vivo microdialysis studies. _Japan J Pharmacol_ 70: 203–205
Article CAS Google Scholar * Scheel-Kruger J, Braestrup C, Nielson M, Golembiowska K, Mogilnicka E . (1976): Cocaine: Discussion on the role of dopamine in the biochemical mechanism of
action. In Ellinwood EH, Kilbey MM (eds), _Cocaine and Other Stimulants._ New York, Plenum Press, pp 373–407 Google Scholar * Schmidt CJ, Fadayel GM, Sullivan CK, Taylor VL . (1992): 5-HT2
receptors exert a state-dependent regulation of dopaminergic function: Studies with MDL 100907 and the amphetamine analogue, 3,4-methylenedioxymethamphetamine. _Eur J Pharmacol_ 223: 65–74
Article CAS Google Scholar * Sills TL, Greenshaw AJ, Baker GB, Fletcher PS . (1999a): Acute fluoxetine treatment potentiates amphetamine-hyperactivity and amphetamine-induced
accumbens–dopamine release: Possible pharmacokinetic interaction. _Psychopharmacology_ 141: 421–427 Article CAS Google Scholar * Sills TL, Greenshaw AJ, Baker GB, Fletcher PS . (1999b):
The potentiating effect of sertraline and fluoxetine on amphetamine-induced locomotor activity is not mediated by serotonin. _Psychopharmacology_ 143: 426–432 Article CAS Google Scholar *
Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ . (1993): Characterization of the 5-HT2 receptor antagonist M100907 as a putative atypical
antipsychotic: Behavioral, electrophysiological, and neurochemical studies. _J Pharmacol Exp Ther_ 266: 684–691 CAS Google Scholar * Steinbush HWM, Niewenhuys R, Verhofstad AAL, van der
Kooy D . (1981): The nucleus raphe dorsalis of the rat and its projection upon the caudate-putamen. A combined cytoarchitectonic, immunocytochemical, and retrograde transport study. _J
Physiol_ 77: 157–174 Google Scholar * Sugrue MF, Shaw G, Charlton KG . (1977): Some effects of mazindol, an anorectic drug, on rat brain monoaminergic systems. _Eur J Pharmacol_ 42: 379–385
Article CAS Google Scholar * Van der Kooy D, Hattori T . (1980): Dorsal raphe cells with collateral projections to the caudate-putamen and substantia niga: A fluorescent retrograde
double labeling study in the rat. _Brain Res_ 186: 1–7 Article CAS Google Scholar * Westfall TC, Tittermary V . (1982): Inhibition of the electrically induced release of [3H]dopamine by
serotonin from superfused rat striatal slices. _Neurosci Lett_ 28: 205–209 Article CAS Google Scholar * Wong DT, Bymaster FP, Reid LR, Threlkeld PG . (1983): Fluoxetine and two other
serotonin uptake inhibitors without affinity for neuronal receptors. _Biochem Pharmacol_ 32: 1287–1293 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This research was
supported by the National Institute on Drug Abuse Grants DA05708, DA06511 (to K.A.C.) and DA 05879 (to L.R.M.). The authors thank Mr. Michael G. Bankson for his thoughtful comments on this
manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pharmacology and Toxicology, The University of Texas Medical Branch, Galveston, TX, USA Lance R McMahon Ph.D &
Kathryn A Cunningham Ph.D Authors * Lance R McMahon Ph.D View author publications You can also search for this author inPubMed Google Scholar * Kathryn A Cunningham Ph.D View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Kathryn A Cunningham Ph.D. RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE McMahon, L., Cunningham, K. Role of 5-HT2A and 5-HT2B/2C Receptors in the Behavioral Interactions Between Serotonin and Catecholamine Reuptake
Inhibitors. _Neuropsychopharmacol_ 24, 319–329 (2001). https://doi.org/10.1016/S0893-133X(00)00206-2 Download citation * Received: 22 May 2000 * Revised: 01 September 2000 * Accepted: 12
September 2000 * Issue Date: 01 March 2001 * DOI: https://doi.org/10.1016/S0893-133X(00)00206-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS *
Dopamine * Locomotor activity * Serotonin * 5-HT2A Receptors * 5-HT2C Receptors





