- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The clinical onset of both the therapeutic and side effects of antipsychotic drugs can take days/weeks to develop. Therefore, it is likely that adaptive changes in neurotransmission
of key systems may only manifest upon chronic administration. Thus, using _in vivo_ microdialysis we have evaluated the acute and chronic (21 days) effects of the atypical antipsychotic
clozapine on nucleus accumbens (NAcc) dopamine (DA) output in the rat. Clozapine (10 mg/kg p.o.) produced an acute 60% increase in extracellular levels of DA in the shell but not the core
subregion of the NAcc. This clozapine-induced effect was also apparent on day 8 (59% increase) of chronic administration. However, on day 22 (following 21 days chronic administration),
clozapine-induced a significant decrease in extracellular DA levels (44% decrease). Since clozapine possesses significant affinity for the 5-HT2C receptor these clozapine-induced effects
were compared to those of SB-243213, a selective 5-HT2C receptor antagonist. SB-243213 (10 mg/kg p.o.) had no effect on NAcc DA levels either acutely or following 21 days chronic
administration. These data demonstrate that the atypical neuroleptic clozapine is more effective at eliciting changes in the shell _vs_ the core subregion of the NAcc. In contrast, chronic
treatment produces a time-dependent reduction in clozapine-induced DA efflux in the shell subregion. This selective temporal change in dopaminergic neurotransmission may be associated with
the delayed therapeutic onset of antipsychotic activity. However, since SB-243213 had no effect on DA levels in the NAcc, it is likely that 5-HT2C receptor antagonism alone is not the
mechanism by which clozapine exerts is actions. SIMILAR CONTENT BEING VIEWED BY OTHERS DIFFERENTIAL DOPAMINE RELEASE BY PSYCHOSIS-GENERATING AND NON-PSYCHOSIS-GENERATING ADDICTIVE SUBSTANCES
IN THE NUCLEUS ACCUMBENS AND DORSOMEDIAL STRIATUM Article Open access 13 September 2021 EFFECT OF 5-HT2A RECEPTOR ANTAGONISM ON LEVELS OF D2/3 RECEPTOR OCCUPANCY AND ADVERSE BEHAVIORAL
SIDE-EFFECTS INDUCED BY HALOPERIDOL: A SPECT IMAGING STUDY IN THE RAT Article Open access 14 January 2021 REBOUND ACTIVATION OF 5-HT NEURONS FOLLOWING SSRI DISCONTINUATION Article Open
access 12 April 2024 INTRODUCTION Chronic administration of neuroleptics or antipsychotic drugs has been the treatment of choice for schizophrenia for the last four decades. The introduction
of chlorpromazine and related compounds into psychiatric practice revolutionized the pharmacotherapy of schizophrenia. Antipsychotic drugs improve positive symptoms in schizophrenia;
however, it was soon discovered that these compounds also produced a variety of neurological syndromes that can occur within hours or days of onset of treatment. The neurological side
effects such as acute dystonia, akathisia, and Parkinson-like syndrome were designated extrapyramidal side effects (EPS) due to the involvement of the extrapyramidal system. In addition,
prolonged administration (months to years) of antipsychotic drugs resulted in the development of periorial tremor and tardive dyskinesia (for a review see Baldessarini and Tarsey, 1980;
Casey, 1991). Until the discovery of clozapine in the early 1970s, it was believed that therapeutic effect could not be separated from these side effects. Clozapine was the first ‘atypical’
antipsychotic that possessed therapeutic efficacy against the positive symptoms of schizophrenia without inducing the EPS-like side effects. Clozapine has subsequently been followed (but
arguably never superceded in terms of efficacy) by a range of molecules with similar atypical like profiles, for example olanzapine, risperidone, and quetiapine. The nucleus accumbens
(NAcc), the main target of the mesotelencephalic dopamine (DA) system, has been the focus of many theories exploring the chemoarchitectural substrates of schizophrenia and affective
disorders as well as theories of reward and motivation (Pontieri et al, 1995; Heidbreder et al, 1999). The existence of subterritories within the NAcc is widely supported by histochemical,
_in vivo_ neurochemical, electrophysiological as well as morphological and ultrastructural studies (for a review see Zahm, 2000). Specifically, the NAcc can be subdivided into a dorsolateral
core and ventromedial shell. Such a structural compartmentation can be defined by the various neurochemical and cellular features of the NAcc that designate specific afferent and efferent
systems, probably involved in different behavioral functions. For example, the shell responds more sensitively than the core to a variety of stimuli, including drugs of abuse (Pontieri et
al, 1995; Hedou et al, 1999; Heidbreder et al, 1999), restraint and pharmacological stress (Deutch and Cameron, 1992; Horger et al, 1995; Kalivas and Duffy, 1995; King et al, 1997), food
(Bassareo and Di Chiara, 1999), and novelty (Rebec et al, 1997; Rebec, 1998). The shell subregion of the NAcc may also play an important role in the mediation of the therapeutic effects of
antipsychotic drugs. Several studies have shown that all antipsychotic agents increase fos protein immunoreactivity or c-fos mRNA expression in the shell, but only those drugs associated
with extrapyramidal motor symptom side effects, such as haloperidol, stimulate fos expression in the core (Deutch, 1996; Hurley et al, 1996; Werme et al, 2000). Furthermore, evidence
suggests that low doses of the atypical antipsychotics produce larger changes in extracellular DA levels in the shell than in the core of the NAcc (Marcus et al, 1996, 2000; Merchant and
Dorsa, 1993). The ability of atypical antipsychotics to induce responses within the shell subregion is thought to be associated with their ability to preferentially affect DA neurones within
the ventral tegmental area (VTA). The NAcc shell projects to the ventral pallidum, which in turn projects to the VTA, whereas the core subregion primarily connects to the dorsolateral
ventral pallidum, which in turn projects to the subthalamic nucleus and the substantia nigra (Meredith et al, 1992). Acute administration of clozapine or amisulpiride increases the number of
spontaneously active neurones in the VTA but not substantia nigra (Blackburn et al, 2002; Di Giovanni et al, 1998), whereas haloperidol produced a significant and equipotent increase in
spontaneous firing in both VTA and substantia nigra neurones (Blackburn et al, 2002; Goldstein et al, 1993). Several studies have shown that repeated treatment with typical antipsychotics
caused a marked reduction in the number of spontaneously active dopaminergic neurones in both the VTA and substantia nigra (Bunney and Grace, 1978; Chiodo and Bunney, 1983, White and Wang,
1983). However, repeated treatment with atypical antipsychotics produced a selective reduction in dopaminergic neuronal activity in the VTA. Considering that clinical onset of both the
therapeutic and side effects of antipsychotic drugs can take days or weeks to develop, it has been postulated that the time-dependent inactivation of VTA and substantia nigral DA neurones by
prolonged administration of antipsychotic drugs may underlie the delayed onset of antipsychotic pharmacotherapy and tardive dyskinesia, respectively (Chiodo and Bunney, 1983; White and
Wang, 1983). The atypical antipsychotic, clozapine has a rich pharmacology that includes affinity for many receptors including dopamine D1, D2, D3, and D4 receptors, and 5-HT1A, 5-HT2A,
5-HT2C, and 5-HT3 receptors (for a review see Ashby and Wang, 1996). Recent electrophysiological and microdialysis studies suggest that 5-HT2C receptor antagonists produce similar effects to
those reported of clozapine treatment on VTA neuronal activity and extracellular DA levels in NAcc (Di Matteo et al, 2002a, 2002b). Thus, it has been suggested that antagonist or inverse
agonist activity at 5-HT2C receptors may underlie the clinical properties of atypical antipsychotics (Herrick-Davis et al, 2000). The present study has investigated the effect of acute
administration of clozapine on extracellular DA levels in core and shell subregions of the NAcc using _in vivo_ microdialysis in the rat, and compared these effects to those of
5-methyl-1-[[2-[92-methyl-3-pyridyl0oxy]-5-pyridyl]carbamoyl]-6-trifluoromethylindone (SB-243213), a highly selective, brain penetrant, 5-HT2C receptor antagonist (Bromidge et al, 2000; Wood
et al, 2001). Furthermore, we have investigated the effect of chronic administration of both compounds (ie clozapine or SB-243213 for 8 and 21 days) on extracellular DA levels in the shell
subregion of the NAcc. MATERIALS AND METHODS SUBJECTS Two groups of 24 male Sprague–Dawley rats (Charles River UK Ltd) weighing 250–300 g were housed in groups of 4–5 per cage in a
temperature and humidity controlled environment with free access to food (restricted to 20 g/day after surgery) and water. Rats were kept on a 12 h light : dark cycle with lights on at 0700.
All experimental procedures carried out in the present study were within the guidelines of the Animals (Scientific Procedures) Act 1986. SURGICAL PROCEDURE The animals were anesthetized
using a mixture of medetomidine (0.04 ml/100 g s.c.) and fentanyl (0.9 ml/kg i.p.). Once deep anesthesia was obtained, rats were transferred to a stereotaxic frame (David Kopf, Topanga, CA)
with the upper incisor bar set at −3.2 mm below the interaural line. Rats were put on a homeothermic blanket set at 37°C throughout. An incision was made to reveal bregma, from which all
coordinates were taken. Holes were then drilled for four anchor screws, and another for unilateral placement of an intracerebral guide cannula (CMA 11, Biotech, UK) into the shell (12 acute,
24 chronic) or core (12 acute) subregions of the NAcc. The coordinates with respect to bregma were as follows (Paxinos and Watson, 1986): for the shell of the NAcc: +1.2 mm anterior (A) to
bregma; 0.8 mm lateral (L) to the midsagittal sinus; 5.8 mm ventral (V) to the dura surface; for the core of the NAcc: A=+1.2 mm, L=2.0 mm, V=5.8 mm. The dura directly beneath the guide was
broken, and the guide was implanted. Using dental cement, the guide and a tether screw (Presearch Limited, UK) placed posterior to the guide were secured in place, and the wound was sealed.
Anesthesia was reversed using a mixture of atipamezole (0.02 ml/100 g s.c.) and nalbuphine (0.02 ml/100 g s.c.). The rats were monitored until they regained their righting reflex. The
animals were allowed to recover for 1 week before the microdialysis experiment started. CHRONIC DOSING PROTOCOL All animals were treated once daily (between 1000 and 2400) for 22 days with
either clozapine (Tocris, UK; 10 mg/kg), SB-243213 (10 mg/kg), or vehicle (1% methyl cellulose, 2.5% BRIJ 35 (Polyoxyethylene 23 lauryl ether-Sigma, Poole, UK)) orally by gastric gavage (2
ml/kg). The 1st, 8th, and 22nd doses were administered during the course of the microdialysis experiment after collection of basal samples as indicated by the experimental protocol. In
total, 18 h prior to the start of experimentation, the animals were randomly assigned to one of six circular polycarbonate microdialysis cages (∅ 285 mm; H: 355 mm) and left to habituate to
their new environment. _IN VIVO_ MICRODIALYSIS PROCEDURE Before implantation, microdialysis probes (CMA/11, 2 mm active cuprophane membrane length, Biotech, UK) were placed in 70% ethanol,
and perfused at 2–5 μl/min with artificial cerebrospinal fluid (aCSF) containing 125 mM NaCl, 2.5 mM KCl, 1.18 mM MgCl2·6H2O, 1.26 mM CaCl2·2H2O, and 2.0 mM Na2HPO4, adjusted to pH 7.4 with
85% H3PO4 (HPLC grade). Both probe inlet and outlet tubing were attached to a dual quartz lined two-channel liquid swivel (Instech 375/D/22QE, Presearch Ltd, Hitchin, UK) on a low mass
spring counterbalanced arm, which in turn was connected to a gas tight syringe (CMA Exmire 1 ml, Biotech, UK) on a microinfusion pump (Univentor 864, Biotech, UK). Probes were perfused at 1
μl/min for 2 h before sample collection. After this equilibration period, three basal samples were collected at 30-min intervals, before animals were administered with clozapine (10 mg/kg
p.o.) or SB-243213 (3 and 10 mg/kg, p.o. in acute study, 10 mg/kg p.o. in chronic study). Microdialysate samples were collected into glass vials (Chromacol Ltd, UK) containing 5 μl 0.3%
acetic acid for an additional 240-min period. All microdialysate samples were subsequently analyzed for DA content using high-performance liquid chromatography (HPLC). Following the
microdialysis experiment, probes were removed, the guide pin was replaced and animals were returned to their home cages. Animals in acute studies were allowed 7 days recovery between uses
and subsequent treatment was allocated according to a randomized crossover design that ensured that no animal was used more than three times or received the same treatment twice. Animals in
the chronic study were tested in further microdialysis experiments on days 8 and 22 of treatment. CHROMATOGRAPHIC ANALYSIS OF BRAIN MICRODIALYSATES (HPLC-ECD) Chromatographic separations
were performed using a Capcell PAK, Strong Cation Exchange column (5 μm UG80, 1.5 × 150 mm2; Shiseido, Japan). The mobile phase consisted of (mM) 13 Na2HPO4, 87 NaH2PO4, 0.1 EDTA, 5 NaCl,
and 20% methanol buffered to pH 6.0, and was delivered via a Jasco PU-980 HPLC pump (Jasco, Tokyo, Japan) at a flow rate of 0.2 ml/min at a temperature of 40°C. DA was detected via an
electrochemical amperometric detector (Decade, Antec-Leyden, Netherlands) fitted with a 3 mm glassy carbon electrode (VT-03, Antec-Leyden, Netherlands) with a working electrode set at +500
mV _vs_ Ag/AgCl reference. The analog data output was smoothed at 40 Hz before collection (LINK, Antec-Leyden, Netherlands). All data were acquired on a PC using Millennium32 software
(Waters, Milford, MA, USA). Samples (10 μl) were injected via a cooled (4°C) Gilson model 234 autosampler (Gilson, Villiers-le-Bel, France) fitted with a six port rotary valve (Model 7125,
Rheodyne, Berkley, CA, USA) with a 20 μl injection loop. HISTOLOGY At the end of the study rats were killed and brains were removed and stored in 4% paraformaldehyde solution for a minimum
of 7 days. Coronal sections (50 μm) were cut using a cryostat microtome and probe placement was verified. Data from animals with incorrect probe placement were removed from further analysis.
DRUG TREATMENTS Clozapine was purchased from Tocris Cookson (Bristol, UK). SB-243213-A (5-methyl-1-[[2-[92-methyl-3-pyridyl0oxy]-5-pyridyl]carbamoyl]-6-trifluoromethylindone hydrochloride)
was synthesized by Medicinal Chemistry, GlaxoSmithKline (Harlow, UK). Clozapine and SB-243213 were ground with one drop (20–30 μl) BRIJ-35 (Sigma, Poole, Dorset, UK) and suspended in 1%
methylcellulose in water, and dosed periorally (p.o.) at a dose volume of 2 ml/kg. Doses of SB-243213-A were chosen based on the robust effects observed in behavioral and
electrophysiological studies (Wood et al, 2001; Blackburn et al, 2002). Dose of clozapine (10 mg/kg) was chosen based on previous in house studies showing robust effects on DA levels in
other regions of the brain. Reasonable 5-HT2C receptor occupancy at this dose was concluded based on evidences of Di Matteo et al (2002a). DATA ANALYSIS All data were expressed as percentage
of baseline control levels. The average concentration of three stable baseline samples was determined and defined as 100%. Total DA efflux was calculated using the trapezoid rule for
determination of area under the curve (AUC). Data were analyzed using repeated measures analysis of variance (ANOVA), followed by _post hoc_ Fishers least significant difference test where
appropriate. The level of significance was set at _P_<0.05. RESULTS EFFECTS OF ACUTE CLOZAPINE (10 MG/KG P.O.) OR SB-243213 (3 AND 10 MG/KG P.O.) ON EXTRACELLULAR LEVELS OF DA IN THE
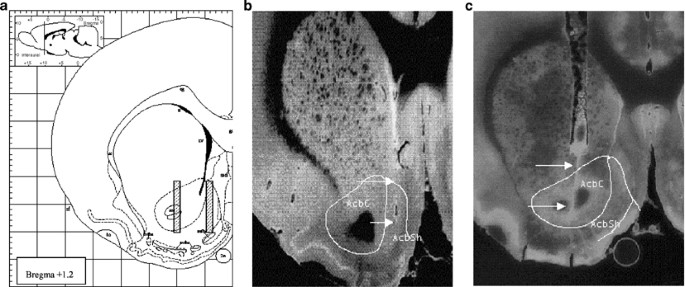
SUBREGIONS OF THE RAT NACC Figure 1 shows (a) schematic representation of the target probe placement and photomicrograph of the probe tracts in the (b) shell and (c) core of the NAcc. Basal
levels of DA within the acute study were not affected by repeated use within the crossover design (Figure 2). In addition, no difference in basal DA levels was observed between NAcc
subregions. Acute clozapine administration produced no effect on DA levels within the core subregion of the NAcc (Figure 3). In contrast, extracellular levels of DA in the shell subregion
were significantly increased by administration of clozapine to a maximum value of 160±22% of preinjection basal levels (F1,7=6.71, _P_<0.05 compared to vehicle). _Post hoc_ analysis
revealed that there was a significant dose × region effect (F1,18=4.406, _P_<0.05). In contrast, treatment with doses of SB-243213, which have previously been shown to be effective in
behavioral models (Wood et al, 2001), produced no change in DA levels in either subregion (Figure 4). EFFECTS OF CHRONIC CLOZAPINE (10 MG/KG P.O, O.D.) OR SB-243213 (10 MG/KG P.O., O.D.) ON
EXTRACELLULAR LEVELS OF DA IN THE SHELL OF THE RAT NACC Chronic treatment with either clozapine or SB-243213 for 7 or 21 days did not affect basal levels of DA (Figure 5). As previously
seen, in the acute subregion comparison, a clozapine challenge to drug naïve animals produced a significant increase in extracellular DA levels in the shell of the NAcc (max 188±43%
F1,11=5.185, _P_=0.0438; Figure 6). Similarly, a clozapine challenge to animals on day 8 of chronic clozapine treatment produced a 159±21% increase in extracellular DA levels in the shell.
This increase failed to reach statistical significance compared to vehicle but was not significantly different from the day 1 response. In contrast, a challenge with clozapine, following 21
days chronic administration (ie on day 22), produced a significant reduction in extracellular DA levels compared to chronic vehicle treated animals reaching a maximum effect of only 56% of
preinjection basal levels (F1,10=8.499, _P_=0.0154). This was also significantly different to the response observed after challenges on days 1 and 8 (F1,11=8.794, _P_=0.0128; and
F1,10=12.707, _P_=0.0051, respectively). Overall analysis of chronic clozapine treatment _vs_ time revealed a significant temporal effect of clozapine (F2,112=5.54, _P_<0.05). In
contrast, a challenge dose of SB-243213 (10 mg/kg) following 7 and 21 days of chronic administration produced no change in extracellular DA levels in the shell subregion of the NAcc (Figure
7). DISCUSSION The present study aimed to investigate the effect of the atypical antipsychotic clozapine on extracellular levels of DA in the shell and core subregions of the NAcc as
measured by microdialysis in the rat and subsequently, whether the acute responses observed were altered following chronic administration. Furthermore, to test whether the 5-HT2C receptor
affinity of the atypical antipsychotic contributed to the effects observed the clozapine-induced responses were compared to those of the selective 5-HT2C receptor antagonist, SB-243213.
Acute clozapine (10 mg/kg p.o.) produced a selective increase in extracellular DA levels in the shell subregion of the NAcc only. However, chronic administration resulted in a time-dependent
reversal of the acute effects of clozapine producing a 40% decrease in extracellular DA levels in response to a clozapine challenge on day 22. In contrast, SB-243213 had no effect on
extracellular DA levels in either subregion of the NAcc after acute dosing or in the shell subregion following 21 days chronic administration. Many microdialysis studies have demonstrated
that an acute challenge with clozapine produces a significant increase in extracellular DA in the rat NAcc (Ichikawa and Meltzer, 1990; Hernandez and Hoebel, 1995; Nomikos et al, 1994).
However, a specific differential subregion effect has not previously been shown using this methodology. The present data agree with the findings from _in vivo_ voltammetric studies (Marcus
et al, 1996, 2000) showing that low doses of clozapine, and other atypical antipsychotics (ie risperidone, sertindole and quetiapine), that exhibit lower levels of occupancy of DA D2
receptors, elicit greater changes in DA concentrations in the shell compared with the core region of the NAcc. In contrast, typical neuroleptics such as haloperidol and raclopride, which are
characterized by potent DA D2 receptor antagonism, produce larger or similar modifications in DA levels in the core _versus_ the shell subregions (Marcus et al, 1996). A clozapine challenge
to animals that had received 21 days treatment prior to the microdialysis study produced a significant reduction in extracellular DA levels that was maintained for the duration of the
sampling period. Reduction in DA neuronal activity, known as depolarization block, after chronic administration of antipsychotic drugs has been demonstrated using electrophysiological
techniques (for a review see Grace et al, 1997). Typical antipsychotics, such as haloperidol and chlorpromazine, produce an increase in neuronal firing upon acute challenge but a subsequent
decrease in the activity of spontaneously active DA cells in both substantia nigra and ventral tegmental regions following chronic treatment (Bunney and Grace, 1978; White and Wang, 1983; Di
Giovanni et al, 1998). In contrast, chronic administration of the atypical antipsychotics clozapine, thioridazine, molindone, and sulpiride produced a selective reduction in firing in the
VTA and exhibited no, or limited effects, in the substantia nigra (Blackburn et al, 2002; Di Giovanni et al, 1998; Skarsfeldt, 1988; White and Wang, 1983). This time-dependent inactivation
of ventral tegmental and substantia nigra DA neurones by prolonged administration of antipsychotic drugs has been suggested to relate to the delayed onset of antipsychotic efficacy and EPS
like side effects (eg tardive dyskinesia) respectively (White and Wang, 1983). Previous attempts to measure the impact of depolarization block on indices of DA release have failed to yield
consistent results. Thus, reports from microdialysis studies investigating the effects of chronic clozapine on basal DA levels in the NAcc are mixed. The data from the present study agree
with a number of studies reporting that basal DA levels in the NAcc are not altered by chronic clozapine administration (Ichikawa and Meltzer, 1990; Invernizzi et al, 1995), while others
report a significant decrease (Chen et al, 1991). The lack of an effect on basal levels of DA in microdialysis studies is not necessarily indicative of a lack of depolarization block. Many
studies have shown that the dopaminergic system displays remarkable adaptability following manipulations that alter DA function. Evidence suggests that as many as 55% of the remaining active
dopaminergic neurones fire in an irregular bursting pattern following chronic clozapine treatment (White and Wang, 1983). In addition, _in vivo_ data have shown that dopaminergic cells
firing in a bursting pattern can release up to six times more DA than those firing in a nonbursting mode (Gonon, 1988). Therefore, although some studies have shown a reduction in basal DA
levels following the induction of depolarization block (Chen et al, 1991; Moore et al, 1998), it is possible that depolarization block may have occurred irrespective of whether there is
evidence of a reduction in basal DA levels. A direct comparison of these data with those of other investigators should be interpreted with care as caveats associated with differences in
probe placement (ie stereotaxic coordinates), microdialysis techniques, and drug administration can all impact the on data generated. In addition, it has been suggested that the insertion of
the probe shortly after implantation surgery into either the NAcc or the striatum may lesion feedback pathways and subsequently reverse the antipsychotic drug-induced effects (Grace, 1992).
However, there is evidence that depolarization block can be observed using acute implantation of microdialysis probes. Thus, Chen et al (1991) showed a reduction in DA levels in the NAcc
after chronic clozapine treatment that could be reversed by apomorphine in animals implanted with the probe 6 h after the last dose of clozapine. It has also been shown that although acute
implantation of the probe can reverse haloperidol-induced depolarization block in the substantia nigra, chronic guide implantation prior to depolarization block with probe insertion 2–3 h
prior to the study allowed reinstatement of this block (Moore et al, 1998). As the present study was conducted using a chronic implantation protocol, it is possible that the reduction in DA
levels observed in the present study, following a clozapine challenge to chronic clozapine treated animals, was as a consequence of VTA depolarization block. As previously discussed the
ability of atypical antipsychotics to induce responses within the shell subregion is thought to be associated with their ability to preferentially affect DA neurones within the VTA. Indeed,
clozapine, olanzapine, and amisulpiride have been shown to exhibit selective activity on VTA neurones at doses that produce no significant effect on substantia nigra neurones (Blackburn et
al, 2002; Di Giovanni et al, 1998; Stockton and Rasmussen, 1996). However, the specific pharamacological characteristic of the atypical antipsychotic, which leads to this selective effect,
is not clearly understood, as all current drugs have a rich pharmacological profile. Since _in vivo_ electrophysiological data have demonstrated that clozapine behaves in a similar manner to
a 5-HT2C receptor antagonist (Blackburn et al, 2002), and clozapine has significant affinity for this receptor, the apparent selective action of clozapine on the activity of VTA neurones
may be due to its affinity for 5-HT2C receptors (Arnt and Skarsfeldt, 1998). High levels of both 5-HT2A and 5-HT2C receptor binding sites and corresponding mRNA are present in several
forebrain areas including basal ganglia and limbic structures (Pazos and Palacios, 1985). While moderate levels of both 5-HT2A and 5-HT2C receptor mRNA are found in the substantia nigra, the
VTA contains predominantly 5-HT2C receptor mRNA (Eberle-Wang et al, 1997). However, in the present study, acute treatment with the selective 5-HT2C receptor antagonist SB-243213 produced no
changes in DA levels in either shell or core subregion of the NAcc. This is in disagreement with a previous study, which demonstrated that the selective 5-HT2C receptor antagonist SB-242084
increased DA levels in the NAcc (Di Matteo et al, 2002b). However, the coordinates used suggest differences in probe placement that, as demonstrated by the clozapine data, can markedly
affect the observed result, and therefore a direct comparison between studies is difficult. Chronic administration and subsequent challenge with SB-243213 also had no effect on levels of DA
in the NAcc shell. This lack of effect after challenge treatment was somewhat unexpected due to reported electrophysiological data showing a reduction in VTA cell firing following treatment
with SB-243213 that was similar to that seen with clozapine (Blackburn et al, 2002). The reason for this disparity is not entirely clear since the decrease in clozapine-induced VTA firing
does appear to result in reductions in clozapine-induced DA efflux. Interestingly, the reduction in spontaneously active cells after SB-243213 treatment was not reversed by apomorphine
treatment, suggesting that a direct depolarization block is possibly not mediating this effect (Blackburn et al, 2002). However, perhaps the simplest explanation is that the 5-HT2C receptor,
under these experiemental conditions, is playing little role in regulating tonic DA efflux, and hence the effects of clozapine in the present study are not mediated solely by the 5-HT2C
receptor. In summary, the present study demonstrates, for the first time, using _in vivo_ microdialysis that an acute challenge of the atypical antipsychotic, clozapine, produces a subregion
dependent increase in extracellular DA levels in the shell of the rat NAcc, which is reversed following a period of chronic administration. These findings agree with electrophysiological
findings, which demonstrate that clozapine has a preferential action on the mesolimbic projection pathway through a selective action on VTA DA neurones. This temporal delay may be associated
with the delayed therapeutic onset of antipsychotic activity. In addition, from these data it would appear that the selectivity of action on the shell subregion and attenuation of activity
following chronic administration do not appear to be mediated solely through 5-HT2C receptors, as neither acute nor chronic treatment with the selective antagonist SB-243213 produced any
changes in extracellular levels of DA in the rat NAcc. REFERENCES * Arnt J, Skarsfeldt T (1998). Do novel antipsychotics have similar pharmacological characteristics? A review of the
evidence. _Neuropsychopharmacology_ 18: 63–101. Article CAS Google Scholar * Ashby Jr CR, Wang RY (1996). Pharmacological actions of the atypical antipsychotic drug clozapine: a review.
_Synapse_ 24: 349–394. Article CAS Google Scholar * Baldessarini RJ, Tarsey D (1980). Dopamine and the pathophysiology of dyskinesias induced by antipsychotic drugs. _Ann Rev Neurosci_ 3:
23–41. Article CAS Google Scholar * Bassareo V, Di Chiara G (1999). Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments.
_Neuroscience_ 89: 637–641. Article CAS Google Scholar * Blackburn TP, Minabe Y, Middlemiss DN, Shirayama Y, Hashimoto K, Ashby Jr CR (2002). Effect of acute and chronic administration of
the selective 5-HT2C receptor antagonist SB-243213 on midbrain dopamine neurons in the rat: an _in vivo_ extracellular single cell study. _Synapse_ 46: 129–139. Article CAS Google Scholar
* Bromidge SM, Dabbs S, Davies DT, Davies S, Duckworth DM, Forbes IT _et al_ (2000). Biarylcarbomolindones are novel and selective 5-HT(2C) receptor inverse agonists: identification of
5-methyl-1-[[2-[92-methyl-3-pyridyl0oxy]-5-pyridyl]carbamoyl]-6- trifluoromethylindone (SB-243213) as a potential antidepressant/anxiolytic agent. _J Med Chem_ 43: 1123–1134. Article CAS
Google Scholar * Bunney BS, Grace AA (1978). Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. _Life Sci_ 23: 1715–1728. Article CAS
Google Scholar * Casey DE (1991). Neuroleptic drug induced extrapyramindal syndromes and tardive dyskinesia. _Schizophr Res_ 4: 109–121. Article CAS Google Scholar * Chen J, Paredes W,
Gardner EL (1991). Chronic treatment with clozapine selectively decreases basal dopamine release in nucleus accumbens but not in caudate-putamen as measured _in vivo_ by brain microdialysis:
further evidence for depolarization block. _Neurosci Lett_ 122: 127–131. Article CAS Google Scholar * Chiodo LA, Bunney BS (1983). Typical and atypical antipsychotics: differential
effects of chronic administration the activity of A9 and A10 midbrain dopaminergic neurones. _J Neurosci_ 3: 1607–1619. Article CAS Google Scholar * Deutch AY (1996). Sites and mechanisms
of action of antipsychotic drugs as revealed by immediate-early gene expression. In: Csernansky JG (ed). _Antipsychotics, Handbook of Experimental Pharmacology_. Springer: Berlin, 120 pp
117–161. Google Scholar * Deutch AY, Cameron DS (1992). Pharmacological characterisation of dopamine systems in the nucleus accumbens core and shell. _Neuroscience_ 46: 49–56. Article CAS
Google Scholar * Di Giovanni G, Di Mascio M, Di Matteo V, Esposito E (1998). Effects of acute and repeated administration of amisulpiride, a dopamine D2/D3 receptor antagonist, on the
electrical activity of midbrain dopaminergic neurones. _J Pharmacol Exp Ther_ 287: 51–57. CAS PubMed Google Scholar * Di Matteo V, Cacchio M, Di Giulio C, Di Giovanni G, Esposito E
(2002a). Biochemical evidence that the atypical antipsychotic drugs clozapine and risperidone block 5-HT2C receptors _in vivo_. _Pharmacol Biochem Behav_ 71: 607–613. Article CAS Google
Scholar * Di Matteo V, Cacchio M, Di Giulio C, Esposito E (2002b). Role of serotonin2C receptors in the control of brain dopaminergic function. _Pharmacol Biochem Behav_ 71: 727–734.
Article CAS Google Scholar * Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet M-F (1997). Pattern of expression of the serotonergic2C receptor messenger RNA in the basal ganglia of adult
rats. _J Comp Neurol_ 384: 233–247. Article CAS Google Scholar * Goldstein JM, Litwin LC, Sutton EB, Malick JB (1993). Seroquel: electrophysiological profile of a potential atypical
antipsychotic. _Psychopharmacology_ 112: 293–298. Article CAS Google Scholar * Gonon F (1988). Nonlinear relationship between impulse flow and dopamine released by rat midbrain
dopaminergic neurones as studied by _in vivo_ electrochemistry. _Neuroscience_ 24: 19–28. Article CAS Google Scholar * Grace AA (1992). The depolarization block hypothesis of neuroleptic
action: implications for the etiology and treatment of schizophrenia. _J Neural Trans (Suppl)_ 36: 91–131. CAS Google Scholar * Grace AA, Bunney BS, Moore H, Todd CL (1997). Dopamine-cell
depolarization block as a model for the therapeutic actions of antipsychotic drugs. _Trends Neurosci_ 20: 31–37. Article CAS Google Scholar * Hedou G, Feldon J, Heidbreder CA (1999).
Effects of cocaine on dopamine in sub-regions of the rat prefrontal cortex and their efferents to subterritories of the nucleus accumbens. _Eur J Pharmacol_ 372: 143–155. Article CAS
Google Scholar * Heidbreder CA, Hedou G, Feldon J (1999). Behavioural neurochemistry reveals a new functional dichotomy in the shell sub-region of the nucleus accumbens. _Prog
Neuro-Psychopharmacol Biol Psychiatry_ 23: 99–132. Article CAS Google Scholar * Hernandez L, Hoebel BG (1995). Chronic clozapine selectively decreases prefrontal cortex dopamine as shown
by simultaneous cortical, accumbens and striatal microdialysis in freely moving rats. _Psychopharmacol Biochem Behav_ 52: 581–589. Article CAS Google Scholar * Herrick-Davis K, Grinde E,
Teitler M (2000). Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. _J Pharmacol Exp Therap_ 295: 226–232. CAS Google Scholar * Horger BA,
Elsworth JD, Roth RW (1995). Selective increase in dopamine utilisation in the shell subdivision of the nucleus accumbens by the benzodiazepine inverse agonist FG 7142. _J Neurochem_ 65:
770–774. Article CAS Google Scholar * Hurley MJ, Stubbs CM, Jenner P, Marsden CD (1996). Dopamine D3 receptors are not involved in the induction of c-fos mRNA by neuroleptic drugs:
comparison of the dopamine D3 receptor antagonist GR-103691 with typical and atypical neuroleptics. _Eur J Pharmacol_ 318: 283–293. Article CAS Google Scholar * Ichikawa J, Meltzer HY
(1990). The effect of chronic clozapine and haloperidol on basal dopamine release and metabolism in rat striatum and nucleus accumbens studies by _in vivo_ microdialysis. _Eur J Pharmacol_
176: 371–374. Article CAS Google Scholar * Invernizzi R, Pozzi R, Samanin R (1995). Further studies on the effects of chronic clozapine on regional extracellular dopamine levels in the
brain of conscious animals. _Brain Res_ 670: 165–168. Article CAS Google Scholar * Kalivas PW, Duffy P (1995). Selective activation of dopamine transmission in the shell of the nucleus
accumbens by stress. _Brain Res_ 675: 325–328. Article CAS Google Scholar * King D, Zigmond MJ, Finlay JM (1997). Effects of dopamine depletion in the medial prefrontal cortex on the
stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. _Neuroscience_ 77: 141–153. Article CAS Google Scholar * Marcus MM, Nomikos GG, Svensson TH
(1996). Differential actions of typical and atypical antipsychotic drugs on dopamine release in the core and shell of the nucleus accumbens. _Eur Neuropsychopharmacol_ 6: 29–39. Article CAS
Google Scholar * Marcus MM, Nomikos GG, Svensson TH (2000). Effects of atypical antipsychotic drugs on dopamine output in the shell and core of the nucleus accumbens: role of 5-HT2A and
_α_1-adrenoceptor antagonism. _Eur Neuropsychopharmacol_ 10: 245–253. Article CAS Google Scholar * Merchant KM, Dorsa DM (1993). Differential induction of C-Fos gene expression by typical
versus atypical antipsychotics. _Proc Natl Acad Sci USA_ 90: 3447–3451. Article CAS Google Scholar * Meredith GE, Agolia R, Arts MPM, Groenewegen HJ, Zahm DS (1992). Morphological
differences between projection neurons of the core and shell in the nucleus accumbens of the rat. _Neuroscience_ 50: 149–162. Article CAS Google Scholar * Moore H, Todd CL, Grace A
(1998). Striatal extracellular dopamine levels in rats with haloperidol-induced depolarization block of substantia nigra dopamine neurones. _J Neurosci_ 18: 5068–5077. Article CAS Google
Scholar * Nomikos GG, Iurlo M, Andersson JL, Kimura K, Svensson T (1994). Systemic administration of amperozide, a new atypical antipsychotic drug, preferentially increases dopaminerggic
release in the rat medial prefrontal cortex. _Psychopharmacology_ 115: 147–156. Article CAS Google Scholar * Paxinos G, Watson C (1986). _The Rat Brain in Stereotaxic Co-ordinates_.
Academic Press: London. Google Scholar * Pazos A, Palacios JM (1985). Quantitative autoradiographic mapping of serotonin receptors in the rat brain: I. Serotonin-1 receptors. _Brain Res_
346: 205–230. Article CAS Google Scholar * Pontieri FE, Tanda G, Di Chiara G (1995). Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the
‘shell’ as compared with the ‘core’ of the rat nucleus accumbens. _Proc Natl Acad Sci USA_ 92: 12304–12308. Article CAS Google Scholar * Rebec GV (1998). Real-time assessments of dopamine
function during behaviour: single-unit recording, iontophoresis, and fast-scan cyclic voltammetry in awake, unrestrained rats. _Alcohol Clin Exp Res_ 22: 32–40. CAS PubMed Google Scholar
* Rebec GV, Christensen JR, Guerra C, Bardo MT (1997). Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. _Brain Res_ 776:
61–67. Article CAS Google Scholar * Skarsfeldt T (1988). Differential effects after repeated treatment with haloperidol, clozapine, thioridazine and tefludazine on SNC and VTA dopamine
neurones in rats. _Life Sci_ 42: 1037–1044. Article CAS Google Scholar * Stockton ME, Rasmussen K (1996). Electrophysiological effects of olanzapine, a novel atypical antipsychotic, on A9
and A10 dopamine neurons. _Neuropsychopharmacology_ 14: 97–104. Article CAS Google Scholar * Werme M, Ringholm A, Olson L, Brené S (2000). Differential patterns of induction of NGFI-B,
Nor1 and c-fos mRNAs in striatal sub-regions by haloperidol and clozapine. _Brain Res_ 863: 112–119. Article CAS Google Scholar * White FJ, Wang RY (1983). Differential effects of
classical and atypical antipsychotic drugs on A9 and A10 dopamine neurones. _Science_ 225: 1054–1057. Article Google Scholar * Wood MD, Reavill C, Trail B, Wilson A, Stean T, Kennett GA
_et al_ (2001). SB-243213; a selective 5-HT2C receptor inverse agonist with improved anxiolytic profile: lack of tolerance and withdrawal anxiety. _Neuropharmacology_ 41: 186–199. Article
CAS Google Scholar * Zahm DS (2000). An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. _Neurosci
Biobehav Rev_ 24: 85–105. Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neuropharmacology, Psychiatry Centre of Excellence for
Drug Discovery, GlaxoSmithKline, Harlow, UK Claire S Shilliam & Lee A Dawson Authors * Claire S Shilliam View author publications You can also search for this author inPubMed Google
Scholar * Lee A Dawson View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Claire S Shilliam. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shilliam, C., Dawson, L. The Effect of Clozapine on Extracellular Dopamine Levels in the Shell Subregion of the Rat Nucleus
Accumbens is Reversed Following Chronic Administration: Comparison with a Selective 5-HT2C Receptor Antagonist. _Neuropsychopharmacol_ 30, 372–380 (2005).
https://doi.org/10.1038/sj.npp.1300591 Download citation * Received: 26 May 2004 * Revised: 09 September 2004 * Accepted: 10 September 2004 * Published: 24 November 2004 * Issue Date: 01
February 2005 * DOI: https://doi.org/10.1038/sj.npp.1300591 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * clozapine * nucleus accumbens *
dopamine * microdialysis * 5-HT2C receptors