- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
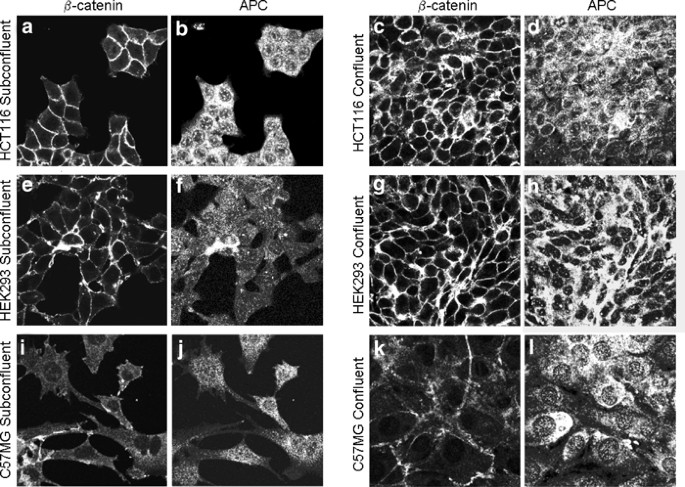
Adenomatous polyposis coli (APC) is a multifunctional tumour suppressor protein, central to development and the mature organism. It is mutated in most cases of colorectal cancer, rendering
it ineffective in mediating β-catenin degradation. We show that localization of full-length APC in colon carcinoma and noncancer cell lines is independent of cell density. However, the
location of truncated APC is a function of cell density and in high-density cells truncated APC is predominantly not nuclear. Although the distribution of truncated APC and β-catenin is
closely linked in subconfluent SW480 cells, at high cell density they are not colocalized. We postulated that in this cell line this could be due to an increase in β-catenin bound to
E-cadherin with formation of adherens junctions at high cell density. However, while in coimmunoprecipitation assays we observe an increase in binding between β-catenin and E-cadherin and a
corresponding decrease in binding between β-catenin and APC at high cell density, we did not observe a strict colocalization of β-catenin and E-cadherin at the membrane of all cells.
We thank Dr IS Näthke, University of Dundee for the kind gift of antibody to APC (M-APC). Melanie L Davies is funded through Tenovus Cancer Research Campaign grant 35141; Gwyndaf T Roberts
was supported by BBSRC grant 35086. J Wakeman was supported by a Royal Society award and North West Cancer Research Fund. Thanks to Alysia Battersby, David Pryce and Ramsay McFarlane for
critical reading of this manuscript.
School of Biological Sciences, University of Wales Bangor, Deiniol Road, Bangor, LL57 2UW, Gwynedd, UK
School of Biological Sciences, University of Liverpool, Crown Street, Liverpool, L69 7ZB, UK
Anyone you share the following link with will be able to read this content:





