- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
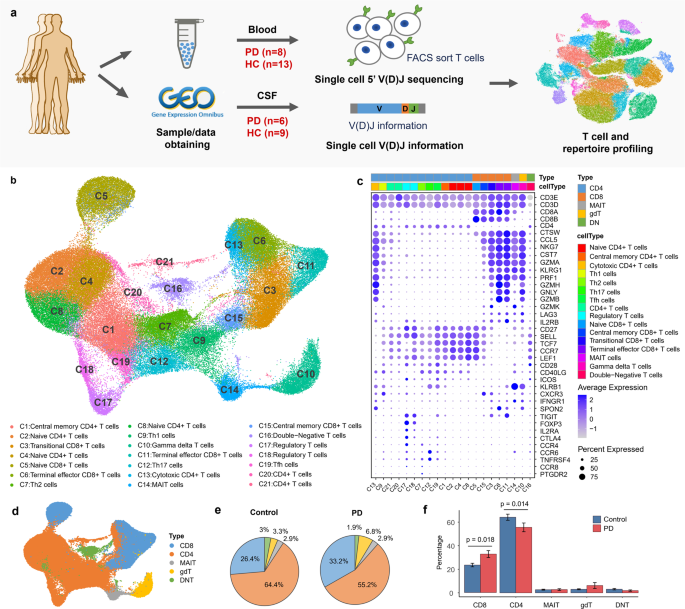
Show authors ABSTRACT Bioprocess development of increasingly challenging therapeutics and vaccines requires a commensurate level of analytical innovation to deliver critical assays across
functional areas. Chromatography hyphenated to numerous choices of detection has undeniably been the preferred analytical tool in the pharmaceutical industry for decades to analyze and
isolate targets (e.g., APIs, intermediates, and byproducts) from multicomponent mixtures. Among many techniques, ion exchange chromatography (IEX) is widely used for the analysis and
purification of biopharmaceuticals due to its unique selectivity that delivers distinctive chromatographic profiles compared to other separation modes (e.g., RPLC, HILIC, and SFC) without
denaturing protein targets upon isolation process. However, IEX method development is still considered one of the most challenging and laborious approaches due to the many variables involved
such as elution mechanism (via salt, pH, or salt-mediated-pH gradients), stationary phase’s properties (positively or negatively charged; strong or weak ion exchanger), buffer type and
ionic strength as well as pH choices. Herein, we introduce a new framework consisting of a multicolumn IEX screening in conjunction with computer-assisted simulation for efficient method
development and purification of biopharmaceuticals. The screening component integrates a total of 12 different columns and 24 mobile phases that are sequentially operated in a
straightforward automated fashion for both cation and anion exchange modes (CEX and AEX, respectively). Optimal and robust operating conditions are achieved via computer-assisted simulation
using readily available software (ACD Laboratories/LC Simulator), showcasing differences between experimental and simulated retention times of less than 0.5%. In addition, automated fraction
collection is also incorporated into this framework, illustrating the practicality and ease of use in the context of separation, analysis, and purification of nucleotides, peptides, and
proteins. Finally, we provide examples of the use of this IEX screening as a framework to identify efficient first dimension (1_D_) conditions that are combined with MS-friendly RPLC
conditions in the second dimension (2_D_) for two-dimensional liquid chromatography experiments enabling purity analysis and identification of pharmaceutical targets. GRAPHICAL ABSTRACT
ACCESS THIS ARTICLE SUBSCRIBE AND SAVE Springer+ Basic $34.99 /Month * Get 10 units per month * Download Article/Chapter or eBook * 1 Unit = 1 Article or 1 Chapter * Cancel anytime Subscribe
now BUY NOW Price excludes VAT (USA) Tax calculation will be finalised during checkout. Instant access to the full article PDF. EXPLORE RELATED SUBJECTS Discover the latest articles and
news from researchers in related subjects, suggested using machine learning. REFERENCES * Regalado EL, Haidar Ahmad IA, Bennett R, D’Atri V, Makarov AA, Humphrey GR, et al. The emergence of
universal chromatographic methods in the research and development of new drug substances. Acc Chem Res. 2019;52(7):1990–2002. Article CAS PubMed Google Scholar * Johnson DE.
Biotherapeutics: challenges and opportunities for predictive toxicology of monoclonal antibodies. Int J Mol Sci. 2018;19(11):3685. Article PubMed Central CAS Google Scholar * Gautam A,
Pan X. The changing model of big pharma: impact of key trends. Drug Discovery Today. 2016;21(3):379–84. Article PubMed Google Scholar * Fekete S, Guillarme D, Sandra P, Sandra K.
Chromatographic, electrophoretic, and mass spectrometric methods for the analytical characterization of protein biopharmaceuticals. Anal Chem. 2016;88(1):480–507. Article CAS PubMed
Google Scholar * Haidar Ahmad IA, Bennett R, Makey D, Shchurik V, Lhotka H, Mann BF, et al. In silico method development for the reversed-phase liquid chromatography separation of proteins
using chaotropic mobile phase modifiers. J Chromatogr B. 2021;1173:122587. Article CAS Google Scholar * Camperi J, Goyon A, Guillarme D, Zhang K, Stella C. Multi-dimensional LC-MS: the
next generation characterization of antibody-based therapeutics by unified online bottom-up, middle-up and intact approaches. Analyst. 2021;146(3):747–69. Article CAS PubMed Google
Scholar * Goyon A, D’Atri V, Bobaly B, Wagner-Rousset E, Beck A, Fekete S, et al. Protocols for the analytical characterization of therapeutic monoclonal antibodies. I – non-denaturing
chromatographic techniques. J Chromatogr B. 2017;1058:73–84. Article CAS Google Scholar * Losacco GL, DaSilva JO, Liu J, Regalado EL, Veuthey J-L, Guillarme D. Expanding the range of
sub/supercritical fluid chromatography: advantageous use of methanesulfonic acid in water-rich modifiers for peptide analysis. J Chromatogr A. 2021;1642:462048. Article CAS PubMed Google
Scholar * Goyon A, Zhang K. Characterization of antisense oligonucleotide impurities by ion-pairing reversed-phase and anion exchange chromatography coupled to hydrophilic interaction
liquid chromatography/mass spectrometry using a versatile two-dimensional liquid chromatography setup. Anal Chem. 2020;92(8):5944–51. Article CAS PubMed Google Scholar * Bennett R, Biba
M, Liu J, Haidar Ahmad IA, Hicks MB, Regalado EL. Enhanced fluidity liquid chromatography: a guide to scaling up from analytical to preparative separations. J Chromatogr A. 2019;1595:190–8.
Article CAS PubMed Google Scholar * Ikegami T. Hydrophilic interaction chromatography for the analysis of biopharmaceutical drugs and therapeutic peptides: a review based on the
separation characteristics of the hydrophilic interaction chromatography phases. J Sep Sci. 2019;42(1):130–213. Article CAS PubMed Google Scholar * Jaag S, Shirokikh M, Lämmerhofer M.
Charge variant analysis of protein-based biopharmaceuticals using two-dimensional liquid chromatography hyphenated to mass spectrometry. J Chromatogr A. 2021;1636:461786. Article CAS
PubMed Google Scholar * Periat A, Fekete S, Cusumano A, Veuthey J-L, Beck A, Lauber M, et al. Potential of hydrophilic interaction chromatography for the analytical characterization of
protein biopharmaceuticals. J Chromatogr A. 2016;1448:81–92. Article CAS PubMed Google Scholar * Piestansky J, Barath P, Majerova P, Galba J, Mikus P, Kovacech B, et al. A simple and
rapid LC-MS/MS and CE-MS/MS analytical strategy for the determination of therapeutic peptides in modern immunotherapeutics and biopharmaceutics. J Pharm Biomed Anal. 2020;189:113449. Article
CAS PubMed Google Scholar * Fekete S, Beck A, Veuthey J-L, Guillarme D. Ion-exchange chromatography for the characterization of biopharmaceuticals. J Pharm Biomed Anal. 2015;113:43–55.
Article CAS PubMed Google Scholar * Abou El Azm N, Fleita D, Rifaat D, Mpingirika EZ, Amleh A, El-Sayed MMH. Production of bioactive compounds from the sulfated polysaccharides extracts
of Ulva lactuca: post-extraction enzymatic hydrolysis followed by ion-exchange chromatographic fractionation. Molecules. 2019;24(11):2132. Article CAS PubMed Central Google Scholar *
Leblanc Y, Bihoreau N, Chevreux G. Characterization of human serum albumin isoforms by ion exchange chromatography coupled on-line to native mass spectrometry. J Chromatogr B.
2018;1095:87–93. Article CAS Google Scholar * McGinnis AC, Cummings BS, Bartlett MG. Ion exchange liquid chromatography method for the direct determination of small ribonucleic acids.
Anal Chim Acta. 2013;799:57–67. Article CAS PubMed Google Scholar * Mommen GPM, Meiring HD, Heck AJR, de Jong APJM. Mixed-bed ion exchange chromatography employing a salt-free pH
gradient for improved sensitivity and compatibility in MudPIT. Anal Chem. 2013;85(14):6608–16. Article CAS PubMed Google Scholar * Bertoletti L, Regazzoni L, Aldini G, Colombo R, Abballe
F, Caccialanza G, et al. Separation and characterisation of beta2-microglobulin folding conformers by ion-exchange liquid chromatography and ion-exchange liquid chromatography–mass
spectrometry. Anal Chim Acta. 2013;771:108–14. Article CAS PubMed Google Scholar * Haidar Ahmad IA, Shchurik V, Nowak T, Mann BF, Regalado EL. Introducing multifactorial peak crossover
in analytical and preparative chromatography via computer-assisted modeling. Anal Chem. 2020;92(19):13443–51. Article CAS PubMed Google Scholar * Ahmed S, Atia NN, Rageh AH. Selectivity
enhanced cation exchange chromatography for simultaneous determination of peptide variants. Talanta. 2019;199:347–54. Article CAS PubMed Google Scholar * Verscheure L, Cerdobbel A,
Sandra P, Lynen F, Sandra K. Monoclonal antibody charge variant characterization by fully automated four-dimensional liquid chromatography-mass spectrometry. J Chromatogr A.
2021;1653:462409. Article CAS PubMed Google Scholar * Gstöttner C, Klemm D, Haberger M, Bathke A, Wegele H, Bell C, et al. Fast and automated characterization of antibody variants with
4D HPLC/MS. Anal Chem. 2018;90(3):2119–25. Article PubMed CAS Google Scholar * Füssl F, Trappe A, Carillo S, Jakes C, Bones J. Comparative elucidation of cetuximab heterogeneity on the
intact protein level by cation exchange chromatography and capillary electrophoresis coupled to mass spectrometry. Anal Chem. 2020;92(7):5431–8. Article PubMed CAS Google Scholar *
Schiavone NM, Bennett R, Hicks MB, Pirrone GF, Regalado EL, Mangion I, et al. Evaluation of global conformational changes in peptides and proteins following purification by supercritical
fluid chromatography. J Chromatogr B. 2019;1110–1111:94–100. Article CAS Google Scholar * Li Z, Wang Q, Wang Y, Wang K, Liu Z, Zhang W, et al. An efficient approach based on basic strong
cation exchange chromatography for enriching methylated peptides with high specificity for methylproteomics analysis. Anal Chim Acta. 2021;1161:338467. Article CAS PubMed Google Scholar
* Patel BA, Pinto NDS, Gospodarek A, Kilgore B, Goswami K, Napoli WN, et al. On-line ion exchange liquid chromatography as a process analytical technology for monoclonal antibody
characterization in continuous bioprocessing. Anal Chem. 2017;89(21):11357–65. Article CAS PubMed Google Scholar * Tsay F-R, Haidar Ahmad IA, Henderson D, Schiavone N, Liu Z, Makarov AA,
et al. Generic anion-exchange chromatography method for analytical and preparative separation of nucleotides in the development and manufacture of drug substances. J Chromatogr A.
2019;1587:129–35. Article CAS PubMed Google Scholar * Yan Y, Liu AP, Wang S, Daly TJ, Li N. Ultrasensitive characterization of charge heterogeneity of therapeutic monoclonal antibodies
using strong cation exchange chromatography coupled to native mass spectrometry. Anal Chem. 2018;90(21):13013–20. Article CAS PubMed Google Scholar * Losacco GL, Wang H, Ahmad IH,
DaSilva J, Makarov AA, Mangion I, et al. Enantioselective UHPLC screening combined with in silico modeling for streamlined development of ultrafast enantiopurity assays. Anal Chem.
2022;94(3):1804–12. Article CAS PubMed Google Scholar * Haidar Ahmad IA, Makey DM, Wang H, Shchurik V, Singh AN, Stoll DR, et al. In silico multifactorial modeling for streamlined
development and optimization of two-dimensional liquid chromatography. Anal Chem. 2021;93(33):11532–9. Article CAS PubMed Google Scholar * Wang H, Herderschee HR, Bennett R, Potapenko M,
Pickens CJ, Mann BF, et al. Introducing online multicolumn two-dimensional liquid chromatography screening for facile selection of stationary and mobile phase conditions in both dimensions.
J Chromatogr A. 2020;1622:460895. Article CAS PubMed Google Scholar * Barhate CL, Joyce LA, Makarov AA, Zawatzky K, Bernardoni F, Schafer WA, et al. Ultrafast chiral separations for
high throughput enantiopurity analysis. Chem Commun. 2017;53(3):509–12. Article CAS Google Scholar * Haidar Ahmad IA, Chen W, Halsey HM, Klapars A, Limanto J, Pirrone GF, et al.
Multi-column ultra-high performance liquid chromatography screening with chaotropic agents and computer-assisted separation modeling enables process development of new drug substances.
Analyst. 2019;144(9):2872–80. Article CAS PubMed Google Scholar * D’Atri V, Murisier A, Fekete S, Veuthey J-L, Guillarme D. Current and future trends in reversed-phase liquid
chromatography-mass spectrometry of therapeutic proteins. TrAC Trends Anal Chem. 2020;130:115962. Article CAS Google Scholar * De Pra M, Greco G, Krajewski MP, Martin MM, George E,
Bartsch N, et al. Effects of titanium contamination caused by iron-free high-performance liquid chromatography systems on peak shape and retention of drugs with chelating properties. J
Chromatogr A. 2020;1611:460619. Article PubMed CAS Google Scholar * Bennett R, Cohen RD, Wang H, Pereira T, Haverick MA, Loughney JW, et al. A selective plate-based assay for trace EDTA
analysis via boron trifluoride-methanol derivatization UHPLC-QqQ-MS/MS enabling biologic and vaccine processes. Anal Chem. 2022;94(3):1678–85. Article CAS PubMed Google Scholar *
Guichard N, Fekete S, Guillarme D, Bonnabry P, Fleury-Souverain S. Computer-assisted UHPLC-MS method development and optimization for the determination of 24 antineoplastic drugs used in
hospital pharmacy. J Pharm Biomed Anal. 2019;164:395–401. Article CAS PubMed Google Scholar * Haidar Ahmad IA, Kiffer A, Barrientos RC, Losacco GL, Singh A, Shchurik V, Wang H, Mangion
I, Regalado EL, et al. In Silico Method Development of Achiral and Chiral Tandem Column Reversed-phase Liquid Chromatography for Multicomponent Pharmaceutical Mixtures. Anal Chem.
2022;94:4065–71. Article CAS PubMed Google Scholar * Makey DM, Shchurik V, Wang H, Lhotka HR, Stoll DR, Vazhentsev A, et al. Mapping the separation landscape in two-dimensional liquid
chromatography: blueprints for efficient analysis and purification of pharmaceuticals enabled by computer-assisted modeling. Anal Chem. 2021;93(2):964–72. Article CAS PubMed Google
Scholar * Bennett R, Haidar Ahmad IA, DaSilva J, Figus M, Hullen K, Tsay F-R, et al. Mapping the separation landscape of pharmaceuticals: rapid and efficient scale-up of preparative
purifications enabled by computer-assisted chromatographic method development. Org Process Res Dev. 2019;23(12):2678–84. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS
The authors would like to thank MRL Postdoctoral Research Fellow Program for sponsoring this research (G.L.L.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Analytical Research and
Development, MRL, Merck & Co., Inc., 126 E. Lincoln Avenue, Rahway, NJ, 07065, USA Gioacchino Luca Losacco, Michael B. Hicks, Jimmy O. DaSilva, Heather Wang, Miraslava Potapenko,
Fuh-Rong Tsay, Imad A. Haidar Ahmad, Ian Mangion & Erik L. Regalado * School of Pharmaceutical Sciences, University of Geneva, CMU, Rue Michel-Servet 1, 1211, Geneva 4, Switzerland Davy
Guillarme * Institute of Pharmaceutical Sciences of Western Switzerland, University of Geneva, CMU, Rue Michel-Servet 1, 1211, Geneva 4, Switzerland Davy Guillarme Authors * Gioacchino Luca
Losacco * Michael B. Hicks * Jimmy O. DaSilva * Heather Wang * Miraslava Potapenko * Fuh-Rong Tsay * Imad A. Haidar Ahmad * Ian Mangion * Davy Guillarme * Erik L. Regalado CORRESPONDING
AUTHORS Correspondence to Gioacchino Luca Losacco or Erik L. Regalado. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS
Reprints and permissions