Play all audios:
ABSTRACT Marine net community production (NCP) tracks uptake of carbon by plankton communities and its potential transport to depth. Relationships between marine microbial community
composition and NCP currently remain unclear despite their importance for assessing how different taxa impact carbon export. We conducted 16 and 18S rRNA gene (rDNA) sequencing on samples
collected across the Western North Atlantic in parallel with high-resolution O2/Ar-derived NCP measurements. Using an internal standard technique to estimate in-situ prokaryotic and
eukaryotic rDNA abundances per liter, we employed statistical approaches to relate patterns of microbial diversity to NCP. Taxonomic abundances calculated using internal standards provided
valuable context to traditional relative abundance metrics. A bloom in the Mid-Atlantic Bight featured high eukaryote abundances with low eukaryotic diversity and was associated with the
harmful algal bloom-forming _Aureococcus anophagefferens_, phagotrophic algae, heterotrophic flagellates, and particle-associated bacteria. These results show that coastal _Aureococcus_
blooms host a distinct community associated with regionally significant peaks in NCP. Meanwhile, weak relationships between taxonomy and NCP in less-productive waters suggest that
productivity across much of this region is not linked to specific microplankton taxa. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY
OTHERS CONTRASTING DIVERSITY PATTERNS OF PROKARYOTES AND PROTISTS OVER TIME AND DEPTH AT THE SAN-PEDRO OCEAN TIME SERIES Article Open access 13 April 2022 INVESTIGATING THE MICROBIAL ECOLOGY
OF COASTAL HOTSPOTS OF MARINE NITROGEN FIXATION IN THE WESTERN NORTH ATLANTIC Article Open access 09 March 2021 BIOGEOGRAPHICAL AND SEASONAL DYNAMICS OF THE MARINE ROSEOBACTER COMMUNITY AND
ECOLOGICAL LINKS TO DMSP-PRODUCING PHYTOPLANKTON Article Open access 14 February 2022 INTRODUCTION Uptake of carbon by phytoplankton and its exchange between organisms in the marine
environment plays a critical role in the carbon cycle, with primary production in the world’s oceans representing half of global net primary production [1]. A small proportion of surface
production [2] is transported to depth via sinking particles, subduction, and other processes, transferring carbon to deep ocean pools with a residence time of millennia or longer [3].
Considerable interest has consequently focused on exploring relationships between surface microbial community structure, marine production [4,5,6], and particulate carbon export [7,8,9]. Net
community production (NCP) rates reflect the productivity and metabolic balance of the surface ocean microbial community. Expressed as the difference between gross primary production and
community respiration, NCP rates estimate the mixed-layer production of organic carbon available for export [10,11,12,13]. NCP patterns have been well-examined independently, as have
patterns of surface ocean community structure. However, direct comparison of relationships between ecology and productivity remains an emerging line of investigation. The Western North
Atlantic is a region of interest for unraveling potential links between community structure and productivity. New production across this region is thought to be driven by a variety of
physical and biological processes including nitrogen fixation, mesoscale features, seasonal mixing, and allochtonous nutrient inputs [14,15,16]. A dominant feature of the Western North
Atlantic is the Sargasso Sea, an oligotrophic region typical of other subtropical gyre systems [17]. While spring and winter phytoplankton blooms occur following winter mixing of nutrients
into the surface layer, the Sargasso Sea in summer exhibits limiting nitrate and phosphate concentrations (_N_ < 50 nmol kg−1, _P_ < 20 nmol kg−1) [18]. Ongoing changes in the
biogeochemistry of the Sargasso Sea may impact community composition, carbon export, and nutrient cycling due to increasing stratification [19] and changing nutrient inputs [20]. Records
suggest gradual community shifts are underway, with haptophyte populations declining and _Synechococcus_ and dinoflagellate groups increasing in abundance [20, 21]. Globally, oligotrophic
subtropical gyres cover some 40% of the planet’s surface [22], and small shifts in microplankton ecology in such regions may have repercussions for biogeochemistry and climate. Large-scale
genomics sampling work has suggested that specific key taxa may be important drivers of carbon export in such regions [7]. To the west, the Western North Atlantic is bounded by the North
American continental shelf. High rates of production are observed along this coast well into summer [23]. In this region, the shelf, shelf break, shelf slope, and Gulf Stream exert dynamic
physical forcings upon resident microplankton, driving variation in community structure and primary production over short transects [24]. Such coastal regions are increasingly being
recognized as potentially important carbon sinks [25] and are also predicted to undergo future ecological shifts in response to eutrophication and climate change [26,27,28]. Considering
ongoing shifts in microplankton community structure in ecosystems across the Western North Atlantic, evaluating the impact of future community shifts upon primary production and potential
carbon export in this region is of great interest. There is thus a need to identify relationships between community composition and NCP. Few regional NCP measurements have been conducted in
the Western N. Atlantic to date, with existing NCP data generally coming from time-series measurements [29] or fine-scale studies [30, 31]. Similarly, while community structure at the
Bermuda Atlantic Time Series (BATS) has been regularly studied [32, 33], broader rDNA amplicon data surveying the whole region are far sparser. In this study, we gathered samples for
high-throughput 16 and 18S rDNA amplicon sequencing and concurrently conducted high-resolution O2/Ar-based NCP measurements using Equilibrator Inlet Mass Spectrometry (EIMS) [34] along three
transects spanning the oligotrophic Sargasso Sea, the Gulf Stream, and the U.S. East Coast. To obtain absolute taxonomic abundances for the sampled communities, we adapted an internal
standard approach for 16 and 18S rDNA sequencing to quantitatively characterize community structure [35]. We then assessed trends in whole-community composition and diversity in relation to
NCP and evaluated associations between productivity and specific microplankton groups identified in our samples. MATERIALS AND METHODS STUDY AREA AND COLLECTION OF O2/AR AND ANCILLARY DATA
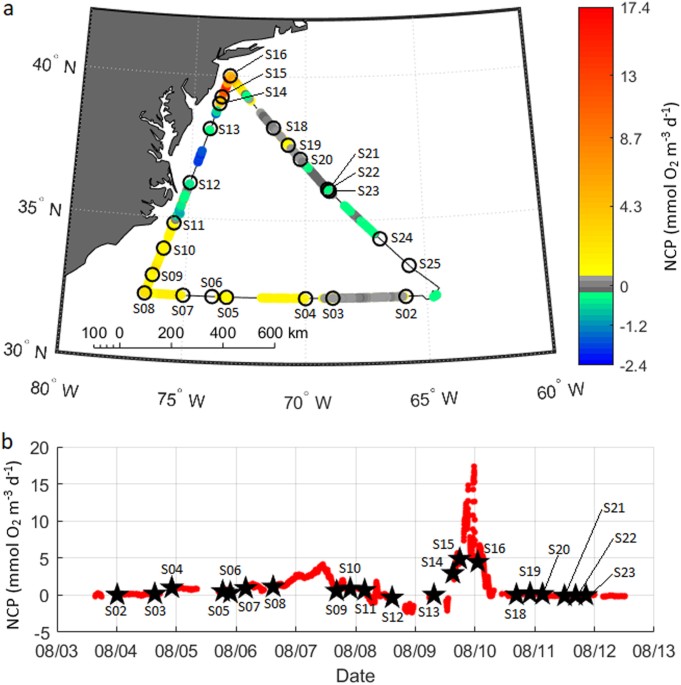
Continuous and discrete measurements were collected over a 3 100 km transect in the western North Atlantic aboard the _R/V Atlantic Explorer_ from 3–12 August 2015. The cruise track
progressed west from the BATS Station (32.3°N, −64.6°W) to the North Carolina coast, then northeast to ~50 km south of Long Island, New York before returning to Bermuda (Fig. 1). Fourteen
CTD casts were conducted during the cruise at 200–400 km intervals. Underway dissolved O2/Ar measurements were collected alongside discrete sampling for chlorophyll and DNA. O2/Ar was
measured continuously from the ship’s underway intake using the EIMS method [34]. Details of O2/Ar-derived NCP calculations and assessment of potential vertical O2/Ar fluxes are described in
the Supplementary Methods. MICROBIAL COMMUNITY SAMPLING AND RDNA AMPLICON SEQUENCING Samples for rDNA analysis were obtained from 5 m CTD casts and underway samples (Table S2) pumped from a
towfish trailing abeam of the vessel at 3–5 m depth. This custom-built towfish, suspended alongside, is trace metal-clean, using plastic tubing and carrying seawater aboard via an
air-driven pump. For each sample, one liter was filtered through a 0.22-μm filter (Millipore, Billerica, MA, USA) using a peristaltic pump, preserved with RNA_later_ (Thermo Fisher, Waltham,
MA, USA), and flash-frozen in liquid nitrogen. At stations with high biomass, the volume of filtrate was reduced to 0.2–0.5 l as filters became clogged. INTERNAL CONTROLS FOR QUANTITATIVE
SEQUENCING A quantitative internal standard approach provides information on per-liter abundance of taxa across samples, yielding more meaningful comparisons between taxonomic abundances and
biological rate measurements. To quantify rDNA copy numbers l−1, internal genomic standards were added to each sample following [35]. Genomic DNA was obtained from the American Type Culture
Collection (ATCC, Manassas, VA, USA) for _Thermus Thermophilus_ (ATCC #27634D-5), a thermophilic hot springs bacterium, and _Schizosaccharomyces pombe_ (ATCC #24843D-5), a yeast species.
The _S. pombe_ genome contains ~110 copies of the 18S V4 rDNA amplicon [35], while the _T. thermophilus_ genome contains two 16S V4 copies [36]. Given the large range in 18S rDNA copy number
across eukaryotic genomes, we determined an appropriate spike of control DNA (0.073 ng) by evaluating the average 18S rDNA concentration in our samples using qPCR with 18S V4 primers. To
ensure that such a diluted spike would reliably manifest in sequencing output, we conducted a pilot sequencing run on duplicate filters from this study at the Boston University Microarray
Core on an Ion Torrent PGM using a 314 chip (Supplementary Methods). The Ion Torrent test revealed that a 15.2 ng _T. thermophilus_ genomic DNA spike resulted in _T. thermophilus_ reads
comprising an average of 5.3% of all reads, while the addition of 0.679 ng of _S. pombe_ gDNA yielded 0.9% _S. pombe_ reads. Based upon these results, we adjusted the spike amounts to
quantities expected to constitute <1% of sequenced reads, adding 0.679 ng of the _S. pombe_ standard and 3.04 ng of the _T. thermophilus_ standard to each sample, both in 50 μl volumes.
This corresponded to adding c.a. 5 780 000 rDNA copies sample−1 of _S. pombe_ and 2 800 000 rDNA copies sample−1 of _T. thermophilus_ genomic DNA. DNA EXTRACTION FOR 16S AND 18S RDNA
SEQUENCING We conducted DNA extraction using the Qiagen DNeasy Plant Mini Kit (Qiagen, Germantown, MD, USA) following manufacturer instructions with slight modifications [37], with internal
gDNA standards added prior to bead-beating [38]. PCR amplification was performed for 30 cycles using custom 16S V4 primers 515F-Y (5′-GTGYCAGCMGCCGCGGTAA-3′) and 805 R
(5′-GACTACNVGGGTATCTAAT-3′) and 18S V4 primers F (5′-CCAGCASCYGCGGTAATTCC-3′) and R (5′-ACTTTCGTTCTTGAT-3′), with attached Illumina adapters and barcodes (Supplementary Table 3). These
primers are adapted from widely-used universal primers for the amplification of marine prokaryotic [39, 40] and eukaryotic [41] taxa, modified to improve coverage of SAR11 and haptophytes
[5, 42]. Primers were each dual-indexed with 6 bp barcodes, using a heterogeneity spacer approach [43, 44]. 16S samples were run at 94 °C for 3 min, 30 cycles at 94 °C for 30 s, 60 °C for 30
s, 72 °C for 1 min, followed by a third stage at 72 °C for 10 min. 18S samples were run identically apart from an annealing temperature of 57 °C. Each 16S PCR reaction (25 μl volume)
consisted of 2.5 μl 10 × PCR buffer, 0.5 μl dNTP mix (10 μM each), 1 μl 50 mM MgSO4, 0.5 μl each of forward and reverse primer (10 μM), 0.1 μl Platinum Taq Hi-Fidelity Polymerase (Thermo
Fisher, Waltham, MA, USA), and 19.4 μl of sterile water. 18S PCR reaction mixtures were identical except polymerase amounts were doubled (0.2 μl per reaction) to address weak amplification,
with a compensating water volume decrease to 19.3 μl. PCR products were purified using the Qiagen QIAquick PCR Purification Kit and quantified using a Qubit 3.0 fluorometer (Life
Technologies, Carlsbad, CA, USA). The samples were then pooled at equimolar concentrations and sequenced using the Illumina MiSeq platform (300 bp PE, V3 chemistry) at the Duke Center for
Genomic and Computational Biology. ANALYSIS PIPELINE We obtained 20 450 700 single-end reads from our 25 sequenced samples. Raw single-end reads were trimmed to remove barcodes, assembled,
and quality filtered following [43] using pandaseq [45]. 16S amplicon length was 296.7 + / − 2.9 bp (mean + / − sd), while mean 18S amplicon length was 424.1 + / − 4.3 bp. Demultiplexing was
performed in QIIME [46]. Five 18S rDNA samples and one 16S rDNA sample contained no reads, the former likely due to a defective forward primer. Primer and other non-biological sequences
were subsequently removed using Tagcleaner [47]. We conducted chimera detection and open-reference OTU picking at 97% similarity using the Usearch 6.1 algorithm [48, 49] and Release 123.1 of
the SILVA database [50]. OTU clustering was performed using the usearch61 method for de novo OTU picking, and the usearch61_ref method for reference-based OTU picking. Alignment was
performed using PyNAST [51] and taxonomy assignment conducted using the RDP classifier 2.2 [52]. Full sequence processing scripts are included in the Supplementary Material. Following
taxonomy assignment, internal standard DNA sequences, eukaryotic metazoans, and plastid 16S sequences were filtered out using the QIIME script ‘filter_taxa_from_otu_table.py’. We further
discarded one sample due to the low volume of filtrate, leaving 19 eukaryotic and 23 prokaryotic samples. Sample diversity metrics were calculated for 16S and 18S datasets using the phyloseq
package [53] for R 3.4.1 [54]. For alpha diversity analyses only, sample libraries were rarefied to the smallest library size in each set of samples (16S: 98 819; 18S: 33 245). Rarefaction
curves begin to level off at the sequencing depths obtained, suggesting that depth was sufficient to represent major patterns of diversity in our samples (Figure S1). Alpha diversity metrics
(observed OTUs and Shannon diversity) were calculated using averages from five rarefactions. Using our non-rarefied sample libraries, calculation of absolute abundances for each OTU was
performed following [38]: $$rDNA\,abundance\,l^{ - 1} = \frac{{\# \,of\,OTU\,reads}}{{R \ast V}}$$ (1) where V is the volume filtered and R represents the recovery ratio of internal
standards (genomic standards sequenced/molecules of genomic standard added). Output OTU tables are included in the Supplementary Material (Supplementary Tables 5a, 5b) Further details of
downstream statistical analyses including ordination and PLS regression are described in the Supplementary Methods. RESULTS AND DISCUSSION PATTERNS OF O2/AR-DERIVED NCP Underway
O2/Ar-derived biological oxygen fluxes within the mixed layer ranged from −2.4 to 17.4 mmol O2 m−3 day−1 (MLD-integrated rates of −25–190 mmol O2 m−2 day−1) (Fig. 1). We observed initial
rates below 0.5 mmol O2 m−3 day−1 in the open ocean, increasing to 1 mmol O2 m−3 day−1 within 400 km of the coast. Turning north, fluxes reached 2–4 mmol O2 m−3 day−1 along the Carolina
coast. Values were subsequently variable along the coast. The highest O2/Ar supersaturation occurred at the expedition’s northernmost extent within a productive phytoplankton bloom, with
values peaking at 17.4 mmol O2 m−3 day−1 south of Long Island. Passing this bloom, O2/Ar supersaturation declined again to typically below 1 mmol O2 m−3 day−1 during transit back to Bermuda.
We assessed the potential contribution of eddy diffusive and entrainment fluxes to mixed-layer O2/Ar values as minimal (Supplementary Methods). Consequently, we report all biological O2
fluxes as NCP rates henceforth. Except when comparing our data with integrated figures from other literature, we also report rates throughout this manuscript as volumetric values, more
suitable for relation to quantitative taxonomic abundances. Overall, our high-resolution NCP measurements agree well with previously measured patterns, with low NCP rates observed in the
open ocean and higher values over the continental shelf along the Mid-Atlantic Bight. The marked peak in productivity at the northern end of the expedition coincided with high measured
nitrogen fixation rates [55] and high Chl a. Peak MLD-integrated productivity, reaching 190 mmol O2 m−2 day−1 (136 mmol C m−2 day−1 assuming a photosynthetic quotient of 1.4 [56]), is of a
similar magnitude as integrated 14C-derived primary production rates for the Mid-Atlantic Bight spring bloom of up to 158 mmol C m−2 day−1 [57]. Our observed rates are also comparable to
summer peak photic-zone primary production of between 145 and 190 mmol C m−2 day−1 modeled for the same area using profile observations [24]. Our low MLD-integrated open-ocean NCP rates,
with a mean of 2.2 mmol O2 m−2 day−1, are also consistent with prior Sargasso Sea O2/Ar-based estimates in September/October of 1.1–3.4 mmol O2 m−2 day−1 [30], as well as modeled summer
regional NCP values of 3–4 mmol O2 m−2 day−1 [58]. MICROBIAL COMMUNITY QUANTITATIVE AND RELATIVE ABUNDANCE PATTERNS Analysis of rDNA reads yielded 7 843 eukaryotic and 5 604 prokaryotic OTUs
across 19 eukaryotic and 23 prokaryotic samples (Supplementary Table 6). 16S and 18S samples contained at least 98 819 and 33 245 reads per sample. Our observations of 16S and 18S rDNA
abundances per liter were within expected bounds. Bacterial 16S rDNA abundances of 1.78 × 108–5.4 × 109 copies l−1 are consistent with bacterial abundances in the Sargasso Sea and Western
North Atlantic of 4.0 × 108–2.3 × 109 cells l−1 [59, 60], assuming a typical 16S copy number of 1–15 [61]. Excluding the three highest NCP stations, where the highest 18S rDNA abundances
were observed (range of 1.43 × 108–3.14 × 1010 18S rDNA genes l−1), the median 18S rDNA abundance was 1.4 × 109 sequences l−1.This is high compared with surface ocean eukaryotic cell
densities of 1 × 107 protists l−1 and 1 × 106 phytoplankton l−1 [62], but is likely driven by variation in 18S rDNA copy number. Peak 18S rDNA abundances, while high, are also reasonable.
_Phaeocystis_ blooms can reach cell counts of 1.5 × 108 cells l−1 [63], and _Aureococcus_ blooms of 6 × 108 cells l−1 have been observed along the Long Island Coast [64]. Absolute abundances
of individual taxa are also consistent with previous observations. For example, the median SAR11 16S rDNA abundance in our samples (Fig. 2b) was 6.2 × 108 rDNA genes l−1 (SAR11 contains one
16S gene copy cell−1), compared with previous measurements of 2 × 108 SAR11 cells l−1 in the Sargasso Sea from fluorescence in-situ hybridization counts [65]. Similarly, we observed a
median of 1.9 × 108 _Prochlorococcus_ 16S rDNA genes l−1 in our samples (Fig. 2b), consistent with Western North Atlantic observations of 1 × 108 cells l−1 based on qPCR quantification and
flow cytometry [66, 67]. Applications of the internal standard approach for samples collected in the lower Amazon River, the Southern Ocean, as well as in soil samples have also demonstrated
good correspondence between the standard-derived abundances and complementary abundance data measured using epifluorescence microscopy, photosynthetic pigments, flow cytometry, phospholipid
fatty acid analysis, and substrate-induced respiration approaches [35, 36, 68]. Notably, calculation of absolute taxonomic abundances using internal standards produces patterns distinct
from those generated using relative abundance metrics (Fig. 3). This is evident among several abundant 18S and 16S OTUs, including SAR11 clade members, as well as the protist clades
_Dinoflagellata_, _Gonyaulacales_, _Alveolata_, and _Gymnodiniphycidae_. The latter four eukaryotes increase in absolute abundance within the bloom environment, while their relative
abundances decrease due to the dominance of _Chrysophyceae_ and _Aureococcus anophagefferens_ within these samples. A similar phenomenon affects SAR11 relative abundances, which are highest
between S2–S9 and S20–S25 due to lower 16S rDNA counts for other prokaryotes at those stations. These discrepancies highlight longstanding criticisms of traditionally-used relative abundance
metrics [36, 38, 69,70,71,72] and illustrate advantages offered by the internal standard approach. In addition, avoidance of issues caused by compositional community data [73, 74] is
valuable when relating taxonomic abundances to microbial or biogeochemical processes like NCP. The internal standard approach is nonetheless subject to several assumptions and limitations. A
key assumption is that recovery rates of DNA standards are comparable to those of natural sequences within the sample. Particularly given the general implications of primer biases in
amplicon work, this premise warrants further investigation. Recovery rate differences due to amplification bias would not alter how the quantitative abundance pattern of a sampled taxon
changes across samples, but might result in discrepancies between estimated and actual in-situ abundances. Another important limitation is that quantitative abundance data produced by this
method remain sensitive to differences in rDNA copy number across taxa. Although better knowledge of 16S copy number variation across prokaryotes has spurred efforts to correct for copy
number differences [75], existing datasets remain limited particularly for eukaryotes, in which rDNA copy number may vary by multiple orders of magnitude. As data collection continues,
corrections will likely become more feasible and commonplace. Among eukaryotes, dinoflagellate lineages dominated all samples except three from the coastal bloom (S14–S16) (Fig. 4a, b). Most
of these dinoflagellate sequences corresponded to _Syndiniales_, alveolate parasites infecting various marine organisms and often detected at high abundances using molecular tools
[76,77,78]. While many of these sequences may originate from endosymbionts inside metazoan zooplankton caught on our filters, _Syndiniales_ also infect microzooplankton protists, including
ciliates, cercozoa, and other dinoflagellates, and clades targeting both host categories often exhibit a short free-living life stage [76]. Consequently, these sequences may also represent
organisms living outside of metazoan hosts, interacting within the marine microbial environment. To a degree, elevated dinoflagellate abundances observed may also reflect high 18S copy
numbers, driven by large dinoflagellate genomes [79, 80]. Two samples (S14, S15) associated with the coastal bloom were dominated (>90% relative abundance) by _Aureococcus
anophagefferens_, a pelagophyte that forms coastal “brown tide” harmful algal blooms (HABs) [81], as well as _Chrysophyceae_ (Fig. 4a, b). qPCR surveys have also detected _A.
anophagefferens_ at low abundances in pelagic waters, which some suggest indicates an oceanic origin for this nuisance algae [82]. A wide distribution of _A. anophagefferens_ is also
supported by our study. We found _Aureococcus_ present in 16 of 19 18S rDNA samples, with a mean of 7.5 × 104 _Aureococcus_ 18S rDNA genes l−1 observed in non-bloom samples. We estimated
abundances of 4.4–6.6 × 104 18S rDNA genes l−1 in open-ocean samples (S24, S25) collected near Bermuda. In comparison, estimated _Aureococcus_ 18S rDNA gene abundances ranged between 1.8 ×
108 and 2.0 × 1010 rDNA genes l−1 within the observed bloom (Fig. 2a). Sample 16 featured a high population (~20%) of _Prymnesiales_, primarily _Chrysochromulina_ and _Chrysoculter_.
_Chrysochromulina_ are another nuisance algae, capable of mixotrophy [83], and forming blooms that can cause fish kills [84]. Other members of _Prymnesiales_ produce harmful hemolytic
compounds [85]. Eukaryotic diversity was lower at two bloom stations, S14 and S15, (Supplementary Figure 2) but was similar across our other samples. Among bacterioplankton, SAR11, SAR86
clade members (appearing as Oceanospirillales in Fig. 4), and _Prochlorococcus_ (Subsection I cyanobacteria) dominated the communities sampled (Fig. 4c, d). The _AEGEAN-169_ clade of
_Alphaproteobacteria_ (Rhodospirillales), as well as MGII _Archaea_ (Thermoplasmatales) also appeared at high proportional abundances. Within the northern bloom, we observed elevated
abundances of _Planctomycetales_, _Flavobacteria, Sphingobacteriales_, and Order III _Cytophagia_, with _Phycisphaerales_ appearing at particularly high abundances (>10%) at two stations.
Not much is currently known about _Phycisphaerales_, although they are hypothesized to form associations with macroalgae, with many representatives facultatively anaerobic [86]. In
addition, these bloom samples also appear to contain more sequences belonging to less-abundant and “rare” taxa (labeled ‘Other’ in Fig. 4). This phenomenon of elevated abundances of “rare”
taxa in bloom events has also been reported elsewhere and may be related to ecological associations with phytoplankton [87, 88]. Bacterial diversity across samples was more uniform than
eukaryotic diversity, with prokaryotic Shannon diversity between 4.1–4.5 versus 2.4–5.7 for eukaryotic samples (Supplementary Figure 2). RELATIONSHIPS BETWEEN MICROBIAL COMMUNITY STRUCTURE
AND NCP At the community level, we observed a negative relationship between measured NCP and eukaryotic Shannon’s H diversity (Pearson: −0.81, Spearman: −0.76, _p_ « 0.01 for both) (Fig. 5),
which was strongly driven by low diversity at two highly productive stations. This relationship does not remain significant with those samples excluded (Pearson: −0.56, Spearman: −0.61, _p_
> 0.01). We observed no relationship between prokaryotic diversity and NCP. Recent debate over the nature of the relationship between marine microplankton diversity and productivity has
been energetic. Any overall relationship between community diversity and productivity would reflect the relative importance of functional diversity, cooperation, competitive exclusion,
selective feeding by grazers, and other factors in governing ecosystem production [6, 89, 90]. Earlier research suggests a peak of phytoplankton diversity at locations with moderate
production, with decreasing diversity observed for less-productive and highly productive sites [91, 92]. Dominance of a handful of taxa beyond the control of grazers may explain decreased
diversity at high productivity. Increased diversity at moderate productivity rates may reflect selective feeding pressures that allow coexistence between a higher diversity of taxa. Within
the Western North Atlantic, our data supports the view that the most productive marine communities may exhibit relatively low eukaryotic diversity, a result consistent with meta-analysis and
model-based findings that the most productive communities are among the least diverse [6]. Principal coordinate analysis (PCoA) of both prokaryotic and eukaryotic samples demonstrated
distinctions between coastal bloom and other samples (Fig. 6), indicating community dissimilarities. Linear regressions of environmental parameters against the first principal component
revealed significant correlations between NCP, temperature, latitude, Chlorophyll, and PC1 for both our 18 and 16S datasets (Supplementary Table 1), suggesting associations between these
parameters and community structure. None of these trends remained significant once data from bloom stations S14, S15, and S16 were excluded, however, indicating that these relationships were
driven largely by these samples, which possess distinctive community structure, high Chl and NCP, and low water temperatures compared to all other stations. RELATIONSHIPS BETWEEN NCP AND
SPECIFIC MICROPLANKTON TAXA Partial Least Squares (PLS) regression analysis revealed groups of prokaryotic and eukaryotic taxa associated with high volumetric NCP rates (Supplementary Tables
4a-4f), with these relationships again strongly driven by the bloom community. Eukaryotic taxa associated with NCP included _Ochrophyta_, _Aureococcus anophagefferens_, picozoa,
cryptophytes, prymnesiophytes, and stramenopiles, such as several uncultured MArine STramenopile (MAST) clades (Fig. 7b). Many of these protists are commonly associated with phytoplankton
bloom conditions. _Aureococcus anophagefferens_ possesses a large genome optimized for uptake of ambient dissolved organic carbon and nitrogen and is adapted for fast growth under turbid,
low-light conditions [81, 93]. Members of _Chrysophyceae_ also form blooms and practice phagotrophy, engulfing, and processing particulate matter [94]. The high abundance of these two taxa
within the bloom implies an environment favoring opportunistic uptake of available particulate and dissolved organic material. Other eukaryotes strongly associated with high NCP include
groups of heterotrophic protists: radiolarians, centrohelids, _Labyrinthulomycetes_, _Ciliophora_, as well as flagellates such as _Kathablepharidae, Choanomonada_, and uncultured marine
stramenopiles. Many of these taxa feed upon algae, bacteria, detritus, and other particles. The associations between these taxa and NCP may indicate flourishing of heterotrophs within an
environment with enhanced food and prey concentrations. The bacterial taxa most correlated with NCP corroborate this picture of a productive bloom ecosystem driven by high phytoplankton
productivity. Groups of _Bacteriodetes_, a class of heterotrophic bacteria generally observed to thrive in particle-rich bloom environments [95], are strongly associated with NCP. Other
bacterial groups primarily exhibiting surface or particle-associated lifestyles, including _Verrucomicrobia_ and _Planctomycetes_, also display high correlations with NCP. Numerous
_Gammaproteobacteria_ taxa, including the fast-growing _Vibrionales_ clade, are also strongly associated with productivity (Fig. 7a). We acknowledge that our community sampling represents a
snapshot of this bloom and cannot capture successional dynamics. 8-day MODIS satellite chlorophyll data measured before and after our cruise suggest that the bloom first appeared in late
July one to two weeks before sampling. Our expedition likely encountered the bloom at its temporal midpoint, with the bloom then fading by late August. We further note that taxa associated
with this event may not be characteristic of other blooms that might occur throughout the region. Although satellite imagery indicates that a large bloom often recurs annually in the
Mid-Atlantic Bight in late summer, additional sampling is required to confirm whether the observed community structure also recurs. Interestingly, when PLS regression analyses were repeated
while excluding bloom stations S14–S16, only a handful of bacterial taxa and eukaryotic taxa remained associated with NCP rates, and the overall strength of associations weakened. Outside of
the observed bloom, moderate correlations with productivity were displayed by just several groups of cryptophytes and bacterioplankton (Fig. 8a, b). These results might indicate that
relationships between specific groups of eukaryotic and prokaryotic taxa and NCP in less-productive locations are either undetected by our study or hidden within the uncertainties of the
measurements conducted. At the same time, such a finding may suggest that links between productivity and community structure in this region are complex, with the abundance of any given taxa
not strongly associated with measured productivity. The relationships we have detailed between productivity and selected microplankton taxa exhibit interesting discrepancies with findings
from similar work conducted in other regions of the global ocean. A TARA Oceans study of associations between bacterial, eukaryotic, and viral taxa, NPP, and particulate carbon export linked
some of the same microplankton groups to primary production and to particle export that were productivity-associated within our full dataset, including _Vibrio_ and _Alteromonadales_ among
bacteria, as well as dinoflagellates, _Labyrinthula_, _Cercozoa_, _Picozoa_, prymnesiophytes, MAST-3, and _Radiolaria_ [7]. Intriguingly, however, many of these abovementioned associations
vanish from our analysis when our dataset is limited to non-bloom station data, whereas Guidi et al. suggest that these same relationships are strong within the oligotrophic ocean. It is
also worth noting that several taxa implicated in carbon export by Guidi et al. show no or even negative correlations with NCP in our analysis, such as _Synechococcus_ (Subsection I
_Cyanobacteria_) and _Oceanospirillales_. Dissimilarities may be attributable to differences in abundance metrics, molecular methods, and the distinctions between in-situ O2/Ar-derived NCP,
modeled NPP, and optically-determined particle export (i.e., not all NCP is exported). Further, ecological dynamics encompassed by our regional study may not be extrapolatable to global
open-ocean data. Yet our work nevertheless spans a considerable area and range of marine biomes. Rather, our results suggest that outside of the observed bloom, productivity across a
relatively wide region is not strongly associated with specific microbial taxa. Such questions warrant further investigation. CONCLUSIONS Our results document a dramatic bloom in
Mid-Atlantic Bight coastal waters, where the harmful algal bloom-forming taxon _Aureococcus_, _Chrysophyceae_, heterotrophic protists, and particle-associated bacterioplankton were strongly
associated with this productivity peak. This result emphasizes the potential significance of large coastal blooms to productivity patterns in the Western North Atlantic, and highlights
HAB-forming _Aureococcus_ as a taxon of particular interest. We also find few associations between taxonomy and NCP across a wide range of less-productive waters, suggesting that specific
microplankton taxa may not be responsible for driving broader patterns of production across much of this region. Our quantitative amplicon sequencing approach serves as a useful tool in
investigating the ocean microbiome and its influence on the marine environment, providing important additional context beyond relative abundance metrics. Coupled with the ever-increasing
resolution and capabilities of in-situ biogeochemical methods, adoption of similar study designs can enable more nuanced examination of the role of the microplankton community across diverse
ocean environments. Supplementary information is available at the ISME Journal’s website. Sequences and metadata are available from the NCBI Sequence Read Archive under accession number
SRP126177. REFERENCES * Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: integrating terrestrial and oceanic components. Science. 1998;281:237–40.
Article CAS PubMed Google Scholar * Buesseler KO. The decoupling of production and particulate export in the surface ocean. Glob Biogeochem Cycles. 1998;12:297–310. Article CAS Google
Scholar * Williams R, Follows M. Ocean dynamics and the carbon cycle: principles and mechanisms. Cambridge, United Kingdom: Cambridge University Press; 2011. Book Google Scholar * Cassar
N, Wright SW, Thomson PG, Trull TW, Westwood KJ, de Salas M, et al. The relation of mixed-layer net community production to phytoplankton community composition in the Southern Ocean. Glob
Biogeochem Cycles. 2015;29:446–62. Article CAS Google Scholar * Lin YJ, Cassar N, Marchetti A, Moreno C, Ducklow H, Li ZC. Specific eukaryotic plankton are good predictors of net
community production in the Western Antarctic Peninsula. Scientific Reports 2017;7. * Vallina SM, Follows MJ, Dutkiewicz S, Montoya JM, Cermeno P, Loreau M. Global relationship between
phytoplankton diversity and productivity in the ocean. Nat Commun. 2014;5:4299. Article CAS PubMed Google Scholar * Guidi L, Chaffron S, Bittner L, Eveillard D, Larhlimi A, Roux S, et
al. Plankton networks driving carbon export in the oligotrophic ocean. Nature. 2016;532:465. Article CAS PubMed PubMed Central Google Scholar * Boyd P, Newton P. Evidence of the
potential influence of planktonic community structure on the interannual variability of particulate organic-carbon flux. Deep-Sea Res Part I-Oceanogr Res Pap. 1995;42:619–39. Article Google
Scholar * Richardson TL, Jackson GA. Small phytoplankton and carbon export from the surface ocean. Science. 2007;315:838–840. Article CAS PubMed Google Scholar * Dugdale RC, Goering
JJ. Uptake of new and regenerated forms of nitrogen in primary productivity. Limnology Oceanography 1967;12:196. Article CAS Google Scholar * Li ZC, Cassar N. A mechanistic model of an
upper bound on oceanic carbon export as a function of mixed layer depth and temperature. Biogeosciences. 2017;14:5015–27. Article CAS Google Scholar * Berger WH, Wefer G. Export
production: seasonality and intermittency, and paleoceanographic implications. Palaeogeogr, Palaeoclimatol, Palaeoecol. 1990;89:245–54. Article Google Scholar * Williams, et al. Group
report: Export productivity from the photic zone. In: Berger WH, Smetacek V, Wefer G, (eds). Productivity of the ocean: present and past. Hoboken, N.J: John Wiley and Sons; 1989. p. 99–115.
PJL Google Scholar * Fawcett SE, Lomas MW, Ward BB, Sigman DM. The counterintuitive effect of summer-to-fall mixed layer deepening on eukaryotic new production in the Sargasso Sea. Glob
Biogeochem Cycles. 2014;28:86–102. Article CAS Google Scholar * Lipschultz F, Bates NR, Carlson CA, Hansell DA. New production in the Sargasso Sea: history and current status. Glob
Biogeochem Cycles. 2002;16:1-1–7. Article Google Scholar * McGillicuddy DJ, Robinson AR, Siegel DA, Jannasch HW, Johnson R, Dickeys T, et al. Influence of mesoscale eddies on new
production in the Sargasso Sea. Nature. 1998;394:263–6. Article CAS Google Scholar * Steinberg DK, Carlson CA, Bates NR, Johnson RJ, Michaels AF, Knap AH. Overview of the US JGOFS Bermuda
Atlantic Time-series Study (BATS): a decade-scale look at ocean biology and biogeochemistry. Deep-Sea Res Pt Ii. 2001;48:1405–47. Article CAS Google Scholar * Lomas MW, Bates NR, Johnson
RJ, Knap AH, Steinberg DK, Carlson CA. Two decades and counting: 24-years of sustained open ocean biogeochemical measurements in the Sargasso Sea. Deep-Sea Res Pt Ii. 2013;93:16–32. Article
CAS Google Scholar * Krause JW,Lomas MW,Nelson DM, Biogenic silica at the Bermuda Atlantic Time-series Study site in the Sargasso Sea: Temporal changes and their inferred controls based
on a 15-year record. Glob Biogeochem Cycles. 2009;23. Article CAS Google Scholar * Lomas MW, Steinberg DK, Dickey T, Carlson CA, Nelson NB, Condon RH, et al. Increased ocean carbon export
in the Sargasso Sea linked to climate variability is countered by its enhanced mesopelagic attenuation. Biogeosciences. 2010;7:57–70. Article CAS Google Scholar * Bates NR, Best MHP,
Neely K, Garley R, Dickson AG, Johnson RJ. Detecting anthropogenic carbon dioxide uptake and ocean acidification in the North Atlantic Ocean. Biogeosciences. 2012;9:2509–22. Article CAS
Google Scholar * Karl DM, Church MJ. Microbial oceanography and the Hawaii Ocean Time-series programme. Nat Rev Microbiol. 2014;12:699–713. Article CAS PubMed Google Scholar * O’Reilly
J, Busch D. Phytoplankton primary production on the northwestern Atlantic shelf. Rapp PV Reun Cons Int Explor Mer. 1984;183:255–68. Google Scholar * Mouw CB, Yoder JA. Primary production
calculations in the Mid-Atlantic Bight, including effects of phytoplankton community size structure. Limnol Oceanogr. 2005;50:1232–43. Article CAS Google Scholar * Bauer JE, Cai W-J,
Raymond PA, Bianchi TS, Hopkinson CS, Regnier PAG. The changing carbon cycle of the coastal ocean. Nature. 2013;504:61–70. Article CAS PubMed Google Scholar * Cai W-J. Estuarine and
coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annu Rev Mar Sci. 2011;3:123–45. Article Google Scholar * Doney SC. The growing human footprint on
coastal and open-ocean biogeochemistry. Science. 2010;328:1512–6. Article CAS PubMed Google Scholar * Lomas MW, Glibert PM, Shiah FK, Smith EM. Microbial processes and temperature in
Chesapeake Bay: current relationships and potential impacts of regional warming. Glob Change Biol. 2002;8:51–70. Article Google Scholar * Brix H, Gruber N, Karl DM, Bates NR. On the
relationships between primary, net community, and export production in subtropical gyres. Deep-Sea Res Pt Ii. 2006;53:698–717. Article Google Scholar * Estapa ML, Siegel DA, Buesseler KO,
Stanley RHR, Lomas MW, Nelson NB. Decoupling of net community and export production on submesoscales. Glob Biogeochem Cycles. 2015;29:1266–82. Article CAS Google Scholar *
Mourino-Carballido B, McGillicuddy DJ. Mesoscale variability in the metabolic balance of the Sargasso Sea. Limnol Oceanogr. 2006;51:2675–89. Article Google Scholar * Treusch AH,
Demir-Hilton E, Vergin KL, Worden AZ, Carlson CA, Donatz MG, et al. Phytoplankton distribution patterns in the northwestern Sargasso Sea revealed by small subunit rRNA genes from plastids.
Isme J. 2012;6:481–92. Article CAS PubMed Google Scholar * Vergin KL, Done B, Carlson CA, Giovannoni SJ. Spatiotemporal distributions of rare bacterioplankton populations indicate
adaptive strategies in the oligotrophic ocean. Aquat Microb Ecol. 2013;71:1–U129. Article Google Scholar * Cassar N, Barnett B, Bender M, Kaiser J, Hamme R, Tilbrook B. Continuous
high-frequency dissolved O-2/Ar measurements by equilibrator inlet mass spectrometry. Anal Chem. 2009;81:1855–64. Article CAS PubMed Google Scholar * Lin Y, Gifford S, Ducklow H,
Schofield O, Cassar N (submitted). Towards quantitative marine microbiome community profiling using internal standards. * Smets W, Leff JW, Bradford MA, McCulley RL, Lebeer S, Fierer N. A
method for simultaneous measurement of soil bacterial abundances and community composition via 16S rRNA gene sequencing. Soil Biol Biochem. 2016;96:145–51. Article CAS Google Scholar *
Moisander PH, Beinart RA, Voss M, Zehr JP. Diversity and abundance of diazotrophic microorganisms in the South China Sea during intermonsoon. Isme J. 2008;2:954–67. Article CAS PubMed
Google Scholar * Satinsky BM, Gifford SM, Crump BC, Moran MA. Use of internal standards for quantitative metatranscriptome and metagenome analysis. Microb Metagenomics, Metatranscriptomics,
Metaproteomics. 2013;531:237–50. Article CAS Google Scholar * Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at
a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108:4516–22. Article CAS PubMed Google Scholar * Walters WA, Caporaso JG, Lauber CL, Berg-Lyons D, Fierer N,
Knight R. PrimerProspector: de novo design and taxonomic analysis of barcoded polymerase chain reaction primers. Bioinformatics. 2011;27:1159–61. Article CAS PubMed PubMed Central Google
Scholar * Stoeck T, Bass D, Nebel M, Christen R, Jones MDM, Breiner HW, et al. Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in
marine anoxic water. Mol Ecol. 2010;19:21–31. Article CAS PubMed Google Scholar * Bradley IM, Pinto AJ, Guest JS. Design and evaluation of illumina MiSeq-compatible, 18S rRNA
gene-specific primers for improved characterization of mixed phototrophic communities. Appl Environ Microbiol. 2016;82:5878–91. Article CAS PubMed PubMed Central Google Scholar *
Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome.
2014;2:6. Article PubMed PubMed Central Google Scholar * Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation
pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–20. Article CAS PubMed PubMed Central Google Scholar *
Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. PANDAseq: PAired-eND assembler for illumina sequences. BMC Bioinformatics 2012;13. Article CAS PubMed PubMed Central Google
Scholar * Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6.
Article CAS PubMed PubMed Central Google Scholar * Schmieder R, Lim YW, Rohwer F, Edwards R. TagCleaner: identification and removal of tag sequences from genomic and metagenomic
datasets. BMC Bioinformatics 2010;11. * Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. Article CAS PubMed Google Scholar * Edgar
RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200. Article CAS PubMed PubMed Central Google
Scholar * Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig WG, Peplies J, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible
with ARB. Nucleic Acids Res. 2007;35:7188–96. Article CAS PubMed PubMed Central Google Scholar * Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a
flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010a;26:266–7. Article CAS PubMed Google Scholar * Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian
classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. Article CAS PubMed PubMed Central Google Scholar * McMurdie
PJ, Holmes S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PloS One 2013; 8. * R Core Team. R: A language and environment for
statistical computing. R Foundation for Statistical Computing: Vienna, Austria; 2017 * Tang W, Wang S, Batista D, Dehairs F, Gifford S, Gonzalez A et al (submitted). Coastal oceans broaden
the biogeography of marine N2 fixation. * Laws EA. Photosynthetic quotients, new production and net community production in the open ocean. Deep-Sea Res Part a-Oceanogr Res Pap.
1991;38:143–67. Article CAS Google Scholar * Falkowski PG, Flagg CN, Rowe GT, Smith SL, Whitledge TE, Wirick CD. The fate of a spring phytoplankton bloom—export or oxidation. Cont Shelf
Res. 1988;8:457–84. Article Google Scholar * Letscher RT, Moore JK. Modest net autotrophy in the oligotrophic ocean. Glob Biogeochem Cycles. 2017;31:699–708. Article CAS Google Scholar
* Malmstrom RR, Kiene RP, Cottrell MT, Kirchman DL. Contribution of SAR11 bacteria to dissolved dimethylsulfoniopropionate and amino acid uptake in the North Atlantic ocean. Appl Environ
Microbiol. 2004;70:4129–35. Article CAS PubMed PubMed Central Google Scholar * Rowe JM, DeBruyn JM, Poorvin L, LeCleir GR, Johnson ZI, Zinser ER, et al. Viral and bacterial abundance
and production in the Western Pacific Ocean and the relation to other oceanic realms. Fems Microbiol Ecol. 2012;79:359–70. Article CAS PubMed Google Scholar * Větrovský T, Baldrian P.
The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PLoS ONE. 2013;8:e57923. Article PubMed PubMed Central CAS Google Scholar
* Kirchman DL. Processes in microbial ecology. New York: Oxford University Press; 2012. Google Scholar * Rutten TPA, Sandee B, Hofman ART. Phytoplankton monitoring by high performance
flow cytometry: a successful approach? Cytom Part A. 2005;64A:16–26. Article Google Scholar * Gobler CJ, Renaghan MJ, Buck NJ. Impacts of nutrients and grazing mortality on the abundance
of Aureococcus anophagefferens during a New York brown tide bloom. Limnol Oceanogr. 2002;47:129–41. Article Google Scholar * Morris RM, Rappe MS, Connon SA, Vergin KL, Siebold WA, Carlson
CA, et al. SAR11 clade dominates ocean surface bacterioplankton communities. Nature. 2002;420:806–10. Article CAS PubMed Google Scholar * Johnson ZI, Zinser ER, Coe A, McNulty NP,
Woodward EMS, Chisholm SW. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science. 2006;311:1737–40. Article CAS PubMed Google Scholar *
Zinser ER, Coe A, Johnson ZI, Martiny AC, Fuller NJ, Scanlan DJ, et al. Prochlorococcus ecotype abundances in the North Atlantic Ocean as revealed by an improved quantitative PCR method.
Appl Environ Microbiol. 2006;72:723–32. Article CAS PubMed PubMed Central Google Scholar * Satinsky BM, Fortunato CS, Doherty M, Smith CB, Sharma S, Ward ND et al. Metagenomic and
metatranscriptomic inventories of the lower Amazon River, May 2011. Microbiome 2015;3. * Farrelly V, Rainey FA, Stackebrandt E. Effect of genome size and rrn gene copy number on pcr
amplification of 16s ribosomal-RNA genes from a mixture of bacterial species. Appl Environ Microbiol. 1995;61:2798–801. Article CAS PubMed PubMed Central Google Scholar * Barber RT,
Hiscock MR. A rising tide lifts all phytoplankton: growth response of other phytoplankton taxa in diatom-dominated blooms. Global Biogeochem Cycles 2006;20. Article CAS Google Scholar *
Gifford SM, Sharma S, Rinta-Kanto JM, Moran MA. Quantitative analysis of a deeply sequenced marine microbial metatranscriptome. Isme J. 2011;5:461–72. Article PubMed Google Scholar *
Moran MA, Satinsky B, Gifford SM, Luo H, Rivers A, Chan L-K, et al. Sizing up metatranscriptomics. ISME J. 2013;7:237–43. Article CAS PubMed Google Scholar * Aitchison J. A new approach
to null correlations of proportions. J Int Assoc Math Geol. 1981;13:175–89. Article Google Scholar * Aitchison J. The statistical-analysis of compositional data. J R Stat Soc Ser
B-Methodol. 1982;44:139–77. Google Scholar * Kembel SW, Wu M, Eisen JA, Green JL. Incorporating 16S gene copy number information improves estimates of microbial diversity and abundance.
PloS Comput Biol 2012;8. Article CAS PubMed PubMed Central Google Scholar * Guillou L, Viprey M, Chambouvet A, Welsh RM, Kirkham AR, Massana R, et al. Widespread occurrence and genetic
diversity of marine parasitoids belonging to Syndiniales (Alveolata). Environ Microbiol. 2008;10:3349–65. Article CAS PubMed Google Scholar * Not F, Gausling R, Azam F, Heidelberg JF,
Worden AZ. Vertical distribution of picoeukaryotic diversity in the Sargasso Sea. Environ Microbiol. 2007;9:1233–52. Article CAS PubMed Google Scholar * Romari K, Vaulot D. Composition
and temporal variability of picoeukaryote communities at a coastal site of the English Channel from 18S rDNA sequences. Limnol Oceanogr. 2004;49:784–98. Article Google Scholar * Figueroa
RI, Cuadrado A, Stuken A, Rodriguez F, Fraga S. Ribosomal DNA organization patterns within the dinoflagellate genus alexandrium as revealed by fish: life cycle and evolutionary implications.
Protist. 2014;165:343–63. Article CAS PubMed Google Scholar * Prokopowich CD, Gregory TR, Crease TJ. The correlation between rDNA copy number and genome size in eukaryotes. Genome.
2003;46:48–50. Article CAS PubMed Google Scholar * Gobler CJ, Lonsdale DJ, Boyer GL. A review of the causes, effects, and potential management of harmful brown tide blooms caused by
Aureococcus anophagefferens (Hargraves et Sieburth). Estuaries. 2005;28:726–49. Article Google Scholar * Popels LC, Cary SC, Hutchins DA, Forbes R, Pustizzi F, Gobler CJ, et al. The use of
quantitative polymerase chain reaction for the detection and enumeration of the harmful alga Aureococcus anophagefferens in environmental samples along the United States East Coast. Limnol
Oceanogr-Methods. 2003;1:92–102. Article Google Scholar * Jones HLJ, Leadbeater BSC, Green JC. Mixotrophy in marine species of chrysochromulina (prymnesiophyceae)– ingestion and digestion
of a small green flagellate. J Mar Biol Assoc U K. 1993;73:283–96. Article Google Scholar * Richardson K. Harmful or exceptional phytoplankton blooms in the marine ecosystem. Adv Mar Biol.
1997;31:301–85. Article Google Scholar * Graneli E, Edvardsen B, Roelke DL, Hagstrom JA. The ecophysiology and bloom dynamics of Prymnesium spp. Harmful Algae. 2012;14:260–70. Article
Google Scholar * Lage OM, Bondoso J. Planctomycetes and macroalgae, a striking association. Front Microbiol 2014;5. * Gilbert JA, Steele JA, Caporaso JG, Steinbruck L, Reeder J, Temperton
B, et al. Defining seasonal marine microbial community dynamics. ISME J. 2012;6:298–308. Article CAS PubMed Google Scholar * Hatosy SM, Martiny JBH, Sachdeva R, Steele J, Fuhrman JA,
Martiny AC. Beta diversity of marine bacteria depends on temporal scale. Ecology. 2013;94:1898–1904. Article PubMed Google Scholar * Cermeño P, Rodríguez-Ramos T, Dornelas M, Figueiras
FG, Marañón E, Teixeira IG, et al. Species richness in marine phytoplankton communities is not correlated to ecosystem productivity. Mar Ecol Progress Ser. 2013;488:1–9. Article Google
Scholar * Goebel NL, Edwards CA, Zehr JP, Follows MJ, Morgan SG. Modeled phytoplankton diversity and productivity in the California Current System. Ecol Model. 2013;264:37–47. Article
Google Scholar * Irigoien X, Huisman J, Harris RP. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature. 2004;429:863–7. Article CAS PubMed Google Scholar * Li
WKW. Macroecological patterns of phytoplankton in the northwestern North Atlantic Ocean. Nature. 2002;419:154–7. Article CAS PubMed Google Scholar * Gobler CJ, Berry DL, Dyhrman ST,
Wilhelm SW, Salamov A, Lobanov AV, et al. Niche of harmful alga Aureococcus anophagefferens revealed through ecogenomics. Proc Natl Acad Sci USA. 2011;108:4352–7. Article CAS PubMed
PubMed Central Google Scholar * Caron DA, Alexander H, Allen AE, Archibald JM, Armbrust EV, Bachy C, et al. Probing the evolution, ecology and physiology of marine protists using
transcriptomics. Nat Rev Microbiol. 2017;15:6–20. Article CAS PubMed Google Scholar * Buchan A, LeCleir GR, Gulvik CA, Gonzalez JM. Master recyclers: features and functions of bacteria
associated with phytoplankton blooms. Nat Rev Microbiol. 2014;12:686–98. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This research was supported by an
NSF-CAREER grant awarded to NC (#1350710) and a Chateaubri and Fellowship awarded to SW. NC was also supported by the "Laboratoire d'Excellence" LabexMER (ANR-10-LABX-19) and
co-funded by a grant from the French government under the program "Investissements d'Avenir". RE was supported by an NSF GRFP award (1106401). We thank the staff of the
Bermuda Institute of Ocean Sciences as well as the crew and technicians of the _R/V Atlantic Explorer_ for their valuable assistance in organizing and conducting our field study. We are also
thankful to Rod Johnson, Bruce Williams, and Natasha McDonald at BIOS for their help with sample shipping and analysis. We are additionally grateful to Karoline Faust for her input on
network approaches and to Geoffrey Smith for his help and for our use of his towfish equipment. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Earth and Ocean Sciences, Duke
University, Durham, USA Seaver Wang, Yajuan Lin, Rachel Eveleth & Nicolas Cassar * Laboratoire des Sciences de l’Environnement Marin (LEMAR), UMR 6539 UBO/CNRS/IRD/IFREMER, Institut
Universitaire Européen de la Mer (IUEM), Brest, France Yajuan Lin & Nicolas Cassar * Department of Marine Sciences, the University of North Carolina at Chapel Hill, Chapel Hill, USA
Scott Gifford * Department of Environmental Sciences, University of Virginia, Virginia, USA Rachel Eveleth Authors * Seaver Wang View author publications You can also search for this author
inPubMed Google Scholar * Yajuan Lin View author publications You can also search for this author inPubMed Google Scholar * Scott Gifford View author publications You can also search for
this author inPubMed Google Scholar * Rachel Eveleth View author publications You can also search for this author inPubMed Google Scholar * Nicolas Cassar View author publications You can
also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Nicolas Cassar. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no
conflict of interest. ADDITIONAL INFORMATION Subject Category: Geomicrobiology and microbial contributions to geochemical cycles ELECTRONIC SUPPLEMENTARY MATERIAL GUIDE - SUPPLEMENTARY
INFORMATION SUPPLEMENTARY METHODS SUPPLEMENTARY FIGURES 16S OTU TABLE 18S OTU TABLE AE15 SHIP DATA SEQUENCE DATA PROCESSING SCRIPTS SUPPLEMENTARY TABLE 1 - REGRESSION COEFFICIENTS - PCOA
SUPPLEMENTARY TABLE 2 - STATION METADATA SUPPLEMENTARY TABLE 3 - MISEQ PRIMER SEQUENCES SUPPLEMENTARY TABLE 4A - PLS CORRELATION COEFFICIENTS FOR 16S TAXA, 4TH RANK SUPPLEMENTARY TABLE 4B -
PLS CORRELATION COEFFICIENTS FOR 18S TAXA, 4TH RANK SUPPLEMENTARY TABLE 5 - SAMPLE AND PRIMER GUIDE - ION TORRENT TEST RUN SUPPLEMENTARY TABLE 6 - READ AND OTU COUNTS RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, S., Lin, Y., Gifford, S. _et al._ Linking patterns of net community production and marine microbial community structure in
the western North Atlantic. _ISME J_ 12, 2582–2595 (2018). https://doi.org/10.1038/s41396-018-0163-4 Download citation * Received: 12 December 2017 * Revised: 03 April 2018 * Accepted: 11
May 2018 * Published: 22 June 2018 * Issue Date: November 2018 * DOI: https://doi.org/10.1038/s41396-018-0163-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative