Play all audios:
ABSTRACT Synchrotron small-angle X-ray scattering (SAXS) was measured from aqueous solutions of polyion complexes (PICs), which were prepared by mixing oppositely charged diblock copolymers
MAPTAC-_b_-PMPC and AMPS-_b_-PMPC. MAPTAC represents polycationic (3-(methacryloylamino)propyl)trimethylammonium chloride, AMPS represents polyanionic sodium
2-(acrylamido)-2-methylpropanesulfonate and PMPC represents hydrophilic poly-zwitterion (2-(methacryloyloxy)ethyl phosphorylcholine). The degrees of polymerization of MAPTAC and AMPS were
selected to be the same for each complex, and the ratio (_R_p) of the polymerization degree for the cationic to neutral chains was changed from 0.27 to 9.5. The aggregation number (_N_agg)
of PIC was determined with light scattering combined with field-flow fractionation (FFF). The inner structures of PIC were determined by analyzing SAXS with the core-corona model. When the
MPC chain was much longer than the charged ones (_R_p=0.27), a star-like spherical conformation was formed. With an increase of _R_p, _N_agg increased, while the spherical form was
maintained up to _R_p=1.0. When _R_p=4.7 and 9.5, the PIC became a worm-like cylinder and vesicle, respectively. Based on the determined structural parameters and aggregation numbers, the
index to describe how the tethered PMPC chains were crowded on the shell was determined. It was observed that the crowding parameter was much smaller than that of poly(ethylene glycol in
spherical core-shell polymeric micelles. SIMILAR CONTENT BEING VIEWED BY OTHERS PREPARATION AND DISTORTED CYLINDRICAL MORPHOLOGY OF BLOCK COPOLYMERS CONSISTING OF FLEXIBLE AND SEMIFLEXIBLE
BLOCKS Article 19 July 2021 MOLECULAR DYNAMICS AND STRUCTURE OF POLYROTAXANE IN SOLUTION Article 27 January 2021 DISCOVERING THE MICELLIZATION OF LINEAR A-_B_-(B-_ALT_-C)2-_B_-A MULTIBLOCK
TERPOLYMERS IN SELECTIVE SOLVENTS Article 21 May 2025 INTRODUCTION Mixing two oppositely charged homo-polyelectrolytes results in polyion complexes (PICS), and their isoelectric mixing
normally results in water-incompatible aggregates, which gradually coagulate to form larger aggregates and eventually precipitates.1, 2 Harada and Kataoka3 reported that when oppositely
charged diblock copolymers H-A and H-C are mixed, where H, A and C represent non-ionic hydrophilic, anionic and cationic chains, the A and C blocks form a water-incompatible complex and the
hydrophilic H chains cover to conceal the A/C complex from water. Thus, secondary coagulation is drastically reduced. In other words, the isoelectric H-A/H-C mixtures take a core-shell type
micelle. In contrast to the case of classical PICs, the aqueous solutions dispersing these micelles are quite stable and their size shows an extremely narrow distribution. Presumably, their
micellar structures are determined by a combination of the polyions and hydrophilic chain lengths and their chemical structures. This type of PIC micelles is denoted by PIC micelles in
distinction from classical PICs prepared from homopolymers. It is interesting that the PIC micellar formation is quite similar to that of amphiphilic block copolymers.4 One of the major
applications of PIC micelles is drug-delivery systems.5 In most cases, poly(ethylene glycol) (PEG) is used as the hydrophilic block with a similar purpose, with micelles made from
amphiphilic block copolymers, that is, biocompatibility and enhancement of blood circulation.6 Thus, PEG has been used in many systems in biological applications and the coined term of
‘pegylation’ has become popular. Recently, several groups have emphasized a drawback of PEG: it can induce an immunological response for frequent dosing. This phenomena was first reported by
Ishida _et al._7, 8 for pegylated liposomes. According to these authors, pegylated liposome was rapidly cleared from the blood and accumulated in the liver when injected twice in the same
rat or mouse at several-day intervals, called the ‘accelerated blood clearance phenomenon’. As an alternative, a photophilic polymer bearing a zwitterion as a side chain such as poly
poly(2-(methacryloyloxy)ethylphosphorylcholine) (pMPC) has been synthesized using the reversible addition-fragmentation chain transfer radical polymerization method.9 According to Morisaku
_et al._,10 each MPC repeating unit is associated with ∼24 water molecules, whose number is much larger than that of PEG, which explains why pMPC is more biocompatible than PEG. In fact,
biological systems use zwitterion-type phospholipids in which an anionic phosphate group is combined with a cationic choline. These phospholipids are the major component of all cell
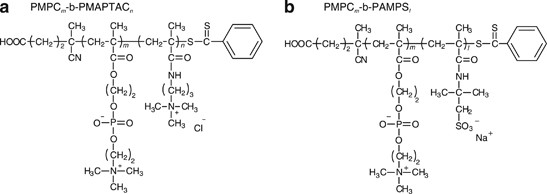
membranes, and these zwitterions form the biological surface of cells. Recently, we synthesized a series of diblock copolymers consisting of an MPC block and either a cationic MAPTAC or
anionic AMPS block using reversible addition-fragmentation chain transfer-controlled radical polymerization. Here, MAPTAC and AMPS represent
poly(3-(methacryloylamino)propyl)trimethylammonium chloride and poly(2-(acrylamido)-2-methylpropanesulfonate), respectively (Figure 1). The mixing of aqueous solutions of oppositely charged
diblock copolymers, pMPC-_b_-pMAPTAC and pMPC-_b_-pAMPS, led to the spontaneous formation of PIC micelles.11 The aim of this study is to characterize these PIC micelles using synchrotron
small-angle X-ray scattering (SAXS) and multi-angle light scattering (MALS) to elucidate the details of the micellar architecture. EXPERIMENTAL PROCEDURE MATERIALS AND MICELLE PREPARATION
Ten samples of pMPC-_b_-pMAPTAC or pMPC-_b_-pAMPS were newly synthesized using a pMPC-based chain-transfer agent for this study. The synthetic details are presented elsewhere.12 The
molecular characters and the sample codes used in this paper are summarized in Table 1, where P, M and A represent pMPC, pMAPTAC and pAMPS, respectively, and the following number indicates
the degree of polymerization for each block. The number-averaged molecular weight was determined using 1H NMR.12 PEG with a molecular weight of 400 g mol−1 was obtained from Wako Pure
Chemical Industries, Ltd (Osaka, Japan) and denoted PEG400. The obtained pMPC-_b_-pMAPTAC and pMPC-_b_-pAMPS were dissolved separately in distillated water containing 0.1 M NaCl (the salt
concentration was the same unless otherwise noted). After being left overnight at room temperature, a pMPC-_b_-pMAPTA solution was added dropwise to a pMPC-_b_-pAMP solution with stirring.
The mixing ratio defined by _f_=[MAPTAC]/([AMPS]+[MAPTAC])) was 0.5 at the isoelectrical composition. We prepared five PIC samples for the present work, and the sample codes and combination
of pMPC-_b_-pMAPTAC and pMPC-_b_-pAMPS are presented in Table 2. Here we introduce the ratio of the polymerization degree of the cationic to neutral chains (_R_p), as indicated in the second
column of the table. FFF COUPLED WITH MALS An Eclipse 3+ separation system (Wyatt Technology Europe, Dernbach, Germany) was used as FFF, which was sequentially connected to a Dawn Heleos II
multiangle static LS detector (Wyatt Technology) and an Optilab rEX DSP differential refractive index (RI) detector (Wyatt Technology) operating at a wavelength of 658 nm, in that order
from the upper stream. A Wyatt channel (Eclipse 3 channel LC) was used, which had a tip-to-tip length of 17.4 cm and a nominal thickness of 250 μm and a membrane (Nadir cellulose membrane 10
kDa LC) was attached to the bottom of the channel. The specific refractive index increment (∂_n/_∂_c_) of the PIC micelles in 0.1 M NaCl aqueous solution was determined for each sample
using a DRM-1021 differential refractometer (Otsuka Electronics, Osaka, Japan). The obtained value was used to determine the molar mass (_M_FFF) for each fraction from the LS intensity using
the Berry plots. The weight-averaged and number-averaged molar masses (_M_w and _M_n) were calculated from RI and _M_FFF fractograms, assuming monodispersity for all fractions. The average
aggregation number _N_agg was determined using the relation of _N_agg=_M_w/(_M_pMPC-b-pMAPTAC+_M_pMPC-b-pANPS), where _M_pMPC-b-pMAPTAC and _M_pMPC-b-pANPS are the number-averaged molecular
weight indicated by each subscript. SYNCHROTRON SAXS MEASUREMENTS SAXS measurements were performed at BL40B2 of SPring-8, Japan.13 A 30 cm × 30 cm imaging plate (Rigaku R-AXIS VII, Tokyo,
Japan) detector was placed at 0.75 or 1.65 m away from the sample. The wavelengths of the incident beam (_λ_) were 0.071 or 0.10 nm. The 0.75 and 1.65 m set-ups provided _q_ ranges of
1.0–8.0 and 0.08–2.0 nm−1, respectively. The irradiation time was fixed at 300 s for all the measurements. A bespoke SAXS vacuum sample chamber14 was used and the X-ray transmittance of the
samples was determined with an ion chamber located in front of the sample and a Si photodiode for the X-ray (Hamamatsu Photonics S8193, Shizuoka, Japan) after the sample. We verified that no
irradiation damage occurred in preliminary experiments. As one of the strongest X-ray sources in the world was being used, we were able to obtain the scattering profiles with a large S/N
even at low concentrations of 1.0 mg ml−1 for C03, C05, C10 and C50, and 0.5 mg ml−1 for C100. In the previous study, we confirmed that the interparticle diffraction, that is, the structural
factor, was negligibly small and, thus, we considered only form factors from scattering objects at these concentrations. The fitting model and its theoretical equations are presented in the
Supplementary Information. RESULTS AND DISCUSSION DETERMINATION OF THE AGGREGATION NUMBER WITH FFF Figure 2 presents the FFF elution fractograms recorded with LS at 90° (upper) and the
concentration determined from refractive index (lower) for three NaCl concentrations ([NaCl]) of C03. The values of _M_FFF determined from MALS are plotted in the upper panel, indicating
that _M_FFF of the 0.1, 0.5 and 1.0 M solutions ranged from 2 to 3 × 106, 2 to 5 × 105 and 3 to 8 × 104, respectively. Here, [NaCl] of the eluting solvent was adjusted to be the same as that
of the sample solution. We injected the same amount of the sample; however, the 0.1 M solution exhibited a much larger peak area than the others. When we calculated the recovery ratio
(defined by the ratio of the injected to eluted amounts of solute), the ratio for the 0.1 M solution was almost 100%, while those of the 0.5 and 1.0 M solutions were 47% and 58%,
respectively, indicating that almost half of the solutes for the 0.5 and 1.0 M solutions did not come out and most likely went through the membrane filter attached to the bottom of the FFF
channel during the focusing or measurement processes. These results can be interpreted as follows: at the higher [NaCl], the polyion parings in PIC were more disturbed by the ion exchange
with sodium or chloride ions and, thus, the unimer/micelle equilibrium shifted to be more favorable for unimer formation. As the size of the unimer was small enough that the unimer was able
to pass through the membrane filter, the recovery decreased with an increase of [NaCl]. As almost 100% recovery was attained at 0.1 M, hereinafter, we performed all the measurements at
[NaCl]=0.1 M. According to Yusa at _et al._,12 dynamic LS revealed a similar ionic strength dependence as that in Figure 2 (see Supplementary Figure 1). The hydrodynamic radii thus obtained
remained constant up to 0.1 M and gradually decreased, eventually reaching <3 nm at [NaCl]>0.9 M. These results indicated that in this high [NaCl], PIC micelles dissociated into
unimers. These results indicated that PIC formed stable aggregates unless [NaCl] exceeded 0.15 M, which is another reason why we performed all the measurements at [NaCl]=0.1 M. Although the
data are not shown, FFF-MALS were performed for the other samples and the calculated _M_w, _M_w/_M_n, and _N_agg are listed in Table 2. For C03: _R_p=0.27, _N_agg was 2.3. For this complex,
the charged chains were much shorter than the neutral one, and the aggregation number is considerably low. Therefore, we can presume that C03 assumes a star-like conformation15 rather than
core-shell-16 or core-corona-17 type micelles. With an increase of _R_p up to 1.0, _N_agg increased. At _R_p=1.0, _N_agg became 30. In the range of _R_p>1.0, different results were
observed. At _R_p=4.7, _N_gg increased to 70 and at _R_p=9.5, _N_agg reached a magnitude of 104. SMALL ANGLE X-RAY SCATTERING Figure 3 presents the SAXS profiles for all of the samples. For
C03, C05 and C10, the exponent _α_ defined by _I_(_q_)∼_q__α_ was ∼0 at low _q_, while _α_∼−2 at high _q_. For C03 and C05, the scattering intensities at _q_<0.1 nm−1 contained parasitic
scattering due to the beam stop. Therefore, we did not use these regions for discussion. With an increase of _R_p, that is, from C03 to C05, the intensity minimum near _q_=0.35 nm−1 became
more obvious and slightly shifted toward lower _q_. For C10, the _q_ dependence of _I_(_q_) at both high and low regions were _α_∼0 and _α_∼−2, respectively; however, there was a clear
minimum at _q_=0.40 nm−1. The value of _α_∼−2 is characteristic for the Gaussian chains, which means that the second term in Supplementary Equation 1 dominates at high _q_ for these three
samples due to relatively small _N_agg. As described above, this second term can be expressed by the Debye function of Supplementary Equation 3, and its asymptotic behaviors at low-_q_ and
high-_q_ are _F__ch_∼_q_0 and ∼_q_−2 (see the Supplementary Information), respectively. Therefore, one may suppose that the scattering profiles for these three samples, especially for C03,
can be fitted by an equation only consisting of the Debye functions and an appropriate model corresponding to such an expression, for example, star polymers without solid core parts.18, 19
However, such a model cannot rationalize the presence of the intensity minimum at _q_=0.35 nm−1. To reproduce both the intensity minimum and the decay of _I_(_q_)∼_q_−2, we need a Debye
function and an additional function that has a larger exponent of _α_, that is, _α<_−2 at high _q_. Thus, we selected Supplementary Equation 4 to fit the data. As _N_agg is relatively
small for C03, C05 and C10, we presumed that this core-corona model is appropriate in terms of physical standpoints. For analyzing the data, we rewrote Supplementary Equation 4 as follows:
Here, _ρ_1, _ρ_2 and _ρ_sol are the electron density of the core, corona and solvent regions, respectively. For _ρ_sol, we used 334 e nm−3 as calculated from the density and composition of
the solvent. _V_1 and _V_2 are the volumes of the core and core-plus-corona region given by _V_i=4/3_πR_i3, respectively. When we fit the data to obtain _R_1, _R_2 and _R__g_, we used the
aggregation number given in Table 2 for _N_agg and treated _ρ_1 and _ρ_2 as adjustable parameters. For the fitting model to make physical sense, we selected the same values of _ρ_1 and _ρ_2
for all three samples, and _R__g_,pMPC <1/2(_R_2−_R_1). The best-fitting curves are presented in Figure 3 as solid lines and are compared with the data, and the used-fitting parameters
are listed in Table 3. The agreement between experiments and the model is sufficient to conclude that the core-corona micelle model well represents the spherical micellar structures of C03,
C05 and C10. With increasing _R_p and thus the increasing _N_agg, the core size increased from 4 to 9 nm. The fully stretched chain lengths of pAMPS were calculated to be 6.2, 12.4 and 24.9
nm, respectively, and these lengths are much longer than the core sizes. This result implies that the polyion pairs are randomly folded in the core, as expected. The values of _R_S−_R_C are
∼6 nm and the values of _R_g,pMPC are 3 nm for all the samples. These unchanged corona sizes are consistent with the length of pMPC not changing for all the samples. The scattering profile
for C50 contained a clear secondary peak at _q_=0.4 nm−1, and there was no Guinier region observed in the present _q_ range. These results indicated that the scattering object for C50 was
much larger than C10, which is consistent with a large _N_agg, and its local structure was well defined to yield the secondary peak. Yusa _et al._12 performed a transmission electron
microscopy examination for C50 and observed short worm-like cylinders (see Supplementary Figure 2). Cylindrical objects are consistent with the low-_q_ behavior of SAXS, where the intensity
appears to become _I_(_q_)∼_q_−2 at _q_<0.1 nm−1. We fit the data using Supplementary Equation 6 and the resultant parameters are summarized in Table 3. Yusa _et al._12 also performed
transmission electron microscopy observations and observed that C100 took a mono-layered vesicle.20 As the SAXS intensity at low-_q_ appears to asymptotically merge into the line of
_I_(_q_)∼_q_−2, the vesicle model is consistent with the SAXS data. To fit the data using Supplementary Equation 5, we need to adjust seven parameters, although some of these parameters are
not independent. Fitting with such multiple parameters may not lead to a unique combination of the parameters. We adopted a contrast-matching technique21 to solve this problem as follows.
CONTRAST MATCHING When we sequentially add a third component that has a relatively large electron density to the solvent and if we can assume that such an addition does not change the
micellar structure, the scattering profile dramatically changes even from the same structures21 because such an addition changes the _ρ_-value for the layer adjacent to the solvent, for the
monolayered vesicles described by Supplementary Equation 5, _ρ_2 and _ρ_4. As depicted in Figure 4 (right), we need to determine three geometric parameters: _R_1, the inner diameter of the
vesicle; _t_corona=_R_2−_R_1=_R_4−_R_3, the thickness of the corona layer; and _t_core=_R_3−_R_2, the thickness of the core layer. Among these parameters, there are rules to follow in terms
of the chemical structures. The electron densities of bulk PEG and water are known to be _ρ_PEG=370 e nm−3 and _ρ_water=334 e nm−3, respectively, and thus, _ρ_sol=_wρ_water+(1−_w_)_ρ_PEG.
Furthermore, we assumed that the added PEG chains did not enter the pMPC domain, and thus, _ρ_Ch=360 e nm−3 for all the experiment that have been determined for the other samples. Figure 4
demonstrates how the scattering profile was changed by the addition of PEG400. On adding PEG to the C100 solution, the secondary peak near _q_=0.3 nm−1 become apparent at 5 and 10 wt%, and
then further addition caused the peak to shift to lower angles and become less apparent at 30 wt%. The addition of PEG400 changed _ρ_sol from 334 to 345 e nm−3 at 30 wt%. For each profile,
we attempted to fit all the data using the same parameters, except for _ρ_sol. The resulting theoretical curves are compared with the data in Figure 4, and the obtained parameters are listed
in Table 4. The agreement between the values is good, indicating that the monolayered vesicle model is sufficient to describe C100. The contrast-matching technique has been used commonly in
neutron scattering, normally changing the composition of H2O/D2O.22 In this case, there is almost no risk that changing the solvent composition alters the conformation or structures of the
solutes. To do the same in SAXS, we must add a third component to the solvent to change its electron density, which may cause a change in the structures of the solutes. As illustrated in
Figure 4 (left), all of the scattering profiles were able to be fitted by the same structural parameters, although the scattering profiles were dramatically changed on adding PEG. This fact
ensures that the added PEG did not cause major changes in the structures as well as proving that the obtained parameters were suitable. OVERCROWDING NATURE OF THE MPC BLOCK The manner in
which the tethered chains are crowding on the flat surface can be described by the chain density (_d_) reduced by the sum of the cross-sectional area of the corona chains per surface area,
where _R_g is the radius of gyration of the isolated chain at its unperturbed state.23 When this index is <4, the tethered chains are not interacting with each other. When this index is
from 4 to 10, the chains cross over with each other and the conformation of each chain deviates from a sphere. In the range of _σ_>10, the chains are more stretched to the direction
normal to the surface. Svaneborg and Pedersen24 extended this concept to the shell of polymeric micelles, and the reduced chain density is given by In this model, each corona chain is
assumed to contact at _R_C+_R_g,pMPC from the center of the core. From the same viewpoint, _σ_ can be expanded to the surface of cylindrical and unilamellar vesicles: where _R_g,pMPC is the
radius of gyration of the pMPC single chain; in our case _R_g,pMPC=3 nm for _M_w∼5000, and _R_C,in and _R_C,out are the inner and outer radii of the core of the vesicle, respectively. We
already know all of the parameters needed to determine the surface chain density _d_ given by the three equations. The obtained _d_-values of pMPC are listed in Table 3; these values
increased from 0.1 to 1.0 with an increase of _N_agg. Compared with the polymeric micelles composed of PEG hydrophilic blocks, the values of _d_ for C03, C05 and C10 were very small.25 It
can be assumed from _d_=0.11 for C03 that the pMPC chain was not crowded to completely cover the core. In fact, the graft density of the pMPC on the core surface was ∼0.02 chains per nm2
(this value increased from 0.011 to 0.029 with an increase of _N_agg), which was quite small, compared with nm−2. If the core is sufficiently hydrophobic, this less-crowded shell may cause
secondary aggregation. The stable micelle formation for the present case can be attributed to the less hydrophobic nature of the core prepared from polyion paring and/or to the chemical
nature of MPC, which can be associated with ∼24 water molecules.9 CONCLUSIONS We performed FFF-MALS and SAXS for five PIC samples with different block lengths. _N_agg increased from 2.3 to
104 with an increase of _R_p from 0.27 to 9.5. The PIC structures were determined by analyzing the SAXS data using an appropriate model. When _R_p=0.27, a star-like spherical conformation
was observed. With an increase of _R_p, the spherical form was maintained up to _R_p=1.0, although the conformation transformed from the star to core-corona type. For _R_p=4.7, the complex
transformed into a worm-like cylinder, and when _R_p=9.5 the complex became a vesicle. Based on the determined structural parameters and the aggregation numbers, the crowding parameter (_d_)
was determined. For all of the samples, _d_ was much smaller than that of PEG. REFERENCES * Tsuchida, E., Abe, K. & Honma, M. Aggregation of polyion complexes between synthetic
polyelectrolytes. _Macromolecules_ 9, 112–117 (1976). Article CAS Google Scholar * Pergushov, D. V., Muller, A. H. E. & Schacher, F. H. Micellar interpolyelectrolyte complexes. _Chem.
Soc. Rev._ 41, 6888–6901 (2012). Article CAS Google Scholar * Harada, A. & Kataoka, K. Chain length recognition: core-shell supramolecular assembly from oppositely charged block
copolymers. _Science_ 283, 65–67 (1999). Article CAS Google Scholar * Hamley, I. W. _The Physics of Block Copolymers_, (Oxford University Press, 1998). Google Scholar * Kataoka, K.,
Harada, A. & Nagasaki, Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. _Adv. Drug Deliv. Rev._ 47, 113–131 (2001). Article CAS
Google Scholar * Amiji, M. & Park, K. Prevention of protein adsorption and platelet adhesion on surfaces by PEO/PPO/PEO triblock copolymers. _Biomaterials_ 13, 682–692 (1992). Article
CAS Google Scholar * Ishida, T., Ichikawa, T., Ichihara, M., Sadzuka, Y. & Kiwada, H. Effect of the physicochemical properties of initially injected liposomes on the clearance of
subsequently injected PEGylated liposomes in mice. _J. Controlled Release_ 95, 403–412 (2004). Article CAS Google Scholar * Ishida, T., Harada, M., Wang, X. Y., Ichihara, M., Irimura, K.
& Kiwada, H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose
liposomes. _J. Controlled Release_ 105, 305–317 (2005). Article CAS Google Scholar * Sugihara, S., Blanazs, A., Armes, S. P., Ryan, A. J. & Lewis, A. L. Aqueous dispersion
polymerization: a new paradigm for in situ block copolymer self-assembly in concentrated solution. _J. Am. Chem. Soc._ 133, 15707–15713 (2011). Article CAS Google Scholar * Morisaku, T.,
Watanabe, J., Konno, T., Takai, M. & Ishihara, K. Hydration of phosphorylcholine groups attached to highly swollen polymer hydrogels studied by thermal analysis. _Polymer (Guildf)_ 49,
4652–4657 (2008). Article CAS Google Scholar * Yusa, S.-i., Yokoyama, Y. & Morishima, Y. Synthesis of oppositely charged block copolymers of poly(ethylene glycol) via reversible
addition−fragmentation chain transfer radical polymerization and characterization of their polyion complex micelles in water. _Macromolecules_ 42, 376–383 (2008). Article Google Scholar *
Nakai, K., Nishiuchi, M., Inoue, M., Ishihara, K., Sanada, Y., Sakurai, K. & Yusa, S. i.− Preparation and characterization of polyion complex micelles with phosphobetaine shells.
_Langmuir_ 29, 9651–9661 (2013). Article CAS Google Scholar * Fujisawa, T., Inoue, K., Oka, T., Iwamoto, H., Uruga, T., Kumasaka, T., Inoko, Y., Yagi, N., Yamamoto, M. & Ueki, T.
Small-angle X-ray scattering station at the SPring-8 RIKEN beamline. _J. Appl. Crystallogr._ 33, 797–800 (2000). Article CAS Google Scholar * Masunaga, H., Ogawa, H., Takano, T., Sasaki,
S., Goto, S., Tanaka, T., Seike, T., Takahashi, S., Takeshita, K., Nariyama, N., Ohashi, H., Ohata, T., Furukawa, Y., Matsushita, T., Ishizawa, Y., Yagi, N., Takata, M., Kitamura, H.,
Sakurai, K., Tashiro, K., Takahara, A., Amamiya, Y., Horie, K., Takenaka, M., Kanaya, T., Jinnai, H., Okuda, H., Akiba, I., Takahashi, I., Yamamoto, K., Hikosaka, M., Sakurai, S., Shinohara,
Y., Okada, A. & Sugihara, Y. Multipurpose soft-material SAXS/WAXS/GISAXS beamline at SPring-8. _Polymer J._ 43, 471–477 (2011). Article CAS Google Scholar * Daoud, D. & Cotton,
J. P. Star shaped polymers:a model for the conformation and its concentration dependence. _J. Phys._ 43, 531–538 (1982). Article CAS Google Scholar * Nakano, M., Matsuoka, H., Yamaoka,
H., Poppe, A. & Richter, D. Sphere to rod transition of micelles formed by amphiphilic diblock copolymers of vinyl ethers in aqueous solution. _Macromolecules_ 32, 697–703 (1999).
Article CAS Google Scholar * De Santis, S., Diana Ladogana, R., Diociaiuti, M. & Masci, G. Pegylated and thermosensitive polyion complex micelles by self-assembly of two oppositely
and permanently charged diblock copolymers. _Macromolecules_ 43, 1992–2001 (2010). Article CAS Google Scholar * Pedersen, J. S. Structure factors effects in small-angle scattering from
block copolymer micelles and star polymers. _J. Chem. Phys._ 114, 2839–2846 (2001). Article CAS Google Scholar * Castelletto, V. & Hamley, I. Small-angle scattering functions of
micelles. _Fibre Diffract. Rev._ 11, 36–43 (2003). Google Scholar * Nakai, K., Ishihara, K. & Yusa, S. Polyion complex vesicles covered with phosphorylcholine groups. _Polym. Prep. Jpn_
62, 522 (2013). Google Scholar * Naruse, K., Eguchi, K., Akiba, I., Sakurai, K., Masunaga, H., Ogawa, H. & Fossey, J. S. Flexibility and cross-sectional structure of an anionic
dual-surfactant wormlike micelle explored with small-angle X-ray scattering coupled with contrast variation technique. _J. Phys. Chem. B_ 113, 10222–10229 (2009). Article CAS Google
Scholar * Potschke, D., Ballauff, M., Lindner, P., Fischer, M. & Vogtle, F. Analysis of the structure of dendrimers in solution by small-angle neutron scattering including contrast
variation. _Macromolecules_ 32, 4079–4087 (1999). Article Google Scholar * Chen, W. Y., Zheng, J. X., Cheng, S. Z. D., Li, C. Y., Huang, P., Zhu, L., Xiong, H., Ge, Q., Guo, Y., Quirk, R.
P., Lotz, B., Deng, L., Wu, C. & Thomas, E. L. Onset of Tethered Chain Overcrowding. _Phys. Rev. Lett._ 93, 028301 (2004). Article Google Scholar * Svaneborg, C. & Pedersen, J. S.
Form factors of block copolymer micelles with excluded-volume interactions of the corona chains determined by monte carlo simulations. _Macromolecules_ 35, 1028–1037 (2001). Article Google
Scholar * Sanada, Y., Akiba, I., Sakurai, K., Shiraishi, K., Yokoyama, M., Mylonas, E., Ohta, N., Yagi, N., Shinohara, Y. & Amemiya, Y. Hydrophobic molecules infiltrating into the
poly(ethylene glycol) domain of the core/shell interface of a polymeric micelle: evidence obtained with anomalous small-angle X-ray scattering. _J. Am. Chem. Soc._ 135, 2574–2582 (2013).
Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was financially supported by the JST CREST program and all the SAXS measurements were performed at SPring-8 40B2
(2011B1735, 2012A1218, 2012B1252, 2012B1662, 2013A1207 and 2013A1594). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemistry and Biochemistry, University of Kitakyushu,
Fukuoka, Japan Shunsuke Sakamoto, Yusuke Sanada, Mizuha Sakashita, Koichi Nishina & Kazuo Sakurai * Structural Materials Science Laboratory SPring-8 Center, RIKEN Harima Institute
Research, Hyogo, Japan Shunsuke Sakamoto, Yusuke Sanada, Mizuha Sakashita & Kazuo Sakurai * Department of Materials Science and Chemistry, Graduate School of Engineering, University of
Hyogo, Hyogo, Japan Keita Nakai & Shin-ichi Yusa Authors * Shunsuke Sakamoto View author publications You can also search for this author inPubMed Google Scholar * Yusuke Sanada View
author publications You can also search for this author inPubMed Google Scholar * Mizuha Sakashita View author publications You can also search for this author inPubMed Google Scholar *
Koichi Nishina View author publications You can also search for this author inPubMed Google Scholar * Keita Nakai View author publications You can also search for this author inPubMed Google
Scholar * Shin-ichi Yusa View author publications You can also search for this author inPubMed Google Scholar * Kazuo Sakurai View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Kazuo Sakurai. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on Polymer Journal website SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION (DOC 1198 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sakamoto, S., Sanada, Y., Sakashita, M. _et al._
Chain-length dependence of polyion complex architecture bearing phosphobetaine block explored using SAXS and FFF-MALS. _Polym J_ 46, 617–622 (2014). https://doi.org/10.1038/pj.2014.25
Download citation * Received: 04 February 2014 * Revised: 13 March 2014 * Accepted: 14 March 2014 * Published: 28 May 2014 * Issue Date: September 2014 * DOI:
https://doi.org/10.1038/pj.2014.25 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * polyion complex * polymer micelle * synchrotron small-angle X-ray
scattering