- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
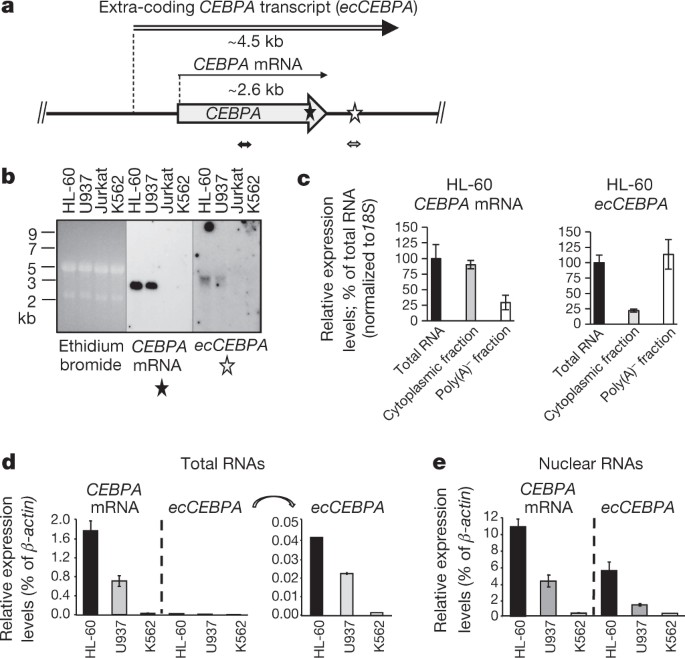
ABSTRACT DNA methylation was first described almost a century ago; however, the rules governing its establishment and maintenance remain elusive. Here we present data demonstrating that
active transcription regulates levels of genomic methylation. We identify a novel RNA arising from the _CEBPA_ gene locus that is critical in regulating the local DNA methylation profile.
This RNA binds to DNMT1 and prevents _CEBPA_ gene locus methylation. Deep sequencing of transcripts associated with DNMT1 combined with genome-scale methylation and expression profiling
extend the generality of this finding to numerous gene loci. Collectively, these results delineate the nature of DNMT1–RNA interactions and suggest strategies for gene-selective
demethylation of therapeutic targets in human diseases. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS NON-CANONICAL FUNCTIONS OF UHRF1 MAINTAIN DNA METHYLATION HOMEOSTASIS IN CANCER
CELLS Article Open access 05 April 2024 THE CONCURRENCE OF DNA METHYLATION AND DEMETHYLATION IS ASSOCIATED WITH TRANSCRIPTION REGULATION Article Open access 06 September 2021 RNA M6A
REGULATES TRANSCRIPTION VIA DNA DEMETHYLATION AND CHROMATIN ACCESSIBILITY Article 07 September 2022 ACCESSION CODES ACCESSIONS GENE EXPRESSION OMNIBUS * GSE32153 * GSE32162 * GSE32168 *
GSE32260 * GSE41279 DATA DEPOSITS Sequencing and microarray data sets are available for download at Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/projects/geo/) under
the following accession numbers: microarray expression, GSE32153; RIPseq, GSE32162; RRBS, GSE32168; and RNAseq, GSE41279. The accession number for the project is GSE32260. REFERENCES *
Jones, P. A. & Baylin, S. B. The fundamental role of epigenetic events in cancer. _Nature Rev. Genet._ 3, 415–428 (2002) Article CAS Google Scholar * Robertson, K. D. DNA methylation,
methyltransferases, and cancer. _Oncogene_ 20, 3139–3155 (2001) Article CAS Google Scholar * Illingworth, R. S. et al. Orphan CpG islands identify numerous conserved promoters in the
mammalian genome. _PLoS Genet._ 6, e1001134 (2010) Article Google Scholar * Saxonov, S., Berg, P. & Brutlag, D. L. A genome-wide analysis of CpG dinucleotides in the human genome
distinguishes two distinct classes of promoters. _Proc. Natl Acad. Sci. USA_ 103, 1412–1417 (2006) Article ADS CAS Google Scholar * Tenen, D. G. Disruption of differentiation in human
cancer: AML shows the way. _Nature Rev. Cancer_ 3, 89–101 (2003) Article CAS Google Scholar * Tada, Y. et al. Epigenetic modulation of tumor suppressor CCAAT/enhancer binding protein α
activity in lung cancer. _J. Natl. Cancer Inst._ 98, 396–406 (2006) Article CAS Google Scholar * Hackanson, B. et al. Epigenetic modification of CCAAT/enhancer binding protein α
expression in acute myeloid leukemia. _Cancer Res._ 68, 3142–3151 (2008) Article CAS Google Scholar * Gupta, R. A. et al. Long non-coding RNA _HOTAIR_ reprograms chromatin state to
promote cancer metastasis. _Nature_ 464, 1071–1076 (2010) Article ADS CAS Google Scholar * Huarte, M. et al. A large intergenic noncoding RNA induced by p53 mediates global gene
repression in the p53 response. _Cell_ 142, 409–419 (2010) Article CAS Google Scholar * Rinn, J. L. et al. Functional demarcation of active and silent chromatin domains in human _HOX_
loci by noncoding RNAs. _Cell_ 129, 1311–1323 (2007) Article CAS Google Scholar * Zhao, J. et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. _Mol. Cell_ 40,
939–953 (2010) Article CAS Google Scholar * Nagano, T. et al. The _Air_ noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. _Science_ 322, 1717–1720 (2008)
Article ADS CAS Google Scholar * Guttman, M. & Rinn, J. L. Modular regulatory principles of large non-coding RNAs. _Nature_ 482, 339–346 (2012) Article ADS CAS Google Scholar *
Ebralidze, A., Wang, Y., Petkova, V., Ebralidse, K. & Junghans, R. P. RNA leaching of transcription factors disrupts transcription in myotonic dystrophy. _Science_ 303, 383–387 (2004)
Article ADS CAS Google Scholar * Poliseno, L. et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. _Nature_ 465, 1033–1038 (2010) Article ADS CAS
Google Scholar * Tay, Y. et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. _Cell_ 147, 344–357 (2011) Article CAS Google Scholar *
Ebisuya, M., Yamamoto, T., Nakajima, M. & Nishida, E. Ripples from neighbouring transcription. _Nature Cell Biol._ 10, 1106–1113 (2008) Article CAS Google Scholar * Preker, P. et al.
RNA exosome depletion reveals transcription upstream of active human promoters. _Science_ 322, 1851–1854 (2008) Article ADS CAS Google Scholar * Ebralidze, A. K. et al. _PU._ _1_
expression is modulated by the balance of functional sense and antisense RNAs regulated by a shared _cis_-regulatory element. _Genes Dev._ 22, 2085–2092 (2008) Article CAS Google Scholar
* Oler, A. J. et al. Human RNA polymerase III transcriptomes and relationships to Pol II promoter chromatin and enhancer-binding factors. _Nature Struct. Mol. Biol._ 17, 620–628 (2010)
Article CAS Google Scholar * Listerman, I., Bledau, A. S., Grishina, I. & Neugebauer, K. M. Extragenic accumulation of RNA polymerase II enhances transcription by RNA polymerase III.
_PLoS Genet._ 3, e212 (2007) Article Google Scholar * Raha, D. et al. Close association of RNA polymerase II and many transcription factors with Pol III genes. _Proc. Natl Acad. Sci. USA_
107, 3639–3644 (2010) Article ADS CAS Google Scholar * Wouters, B. J. et al. Distinct gene expression profiles of acute myeloid/T-lymphoid leukemia with silenced _CEBPA_ and mutations in
_NOTCH1_. _Blood_ 110, 3706–3714 (2007) Article CAS Google Scholar * Meissner, A. et al. Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation
analysis. _Nucleic Acids Res._ 33, 5868–5877 (2005) Article CAS Google Scholar * Leonhardt, H., Page, A. W., Weier, H. U. & Bestor, T. H. A targeting sequence directs DNA
methyltransferase to sites of DNA replication in mammalian nuclei. _Cell_ 71, 865–873 (1992) Article CAS Google Scholar * Hofacker, I. L. Vienna RNA secondary structure server. _Nucleic
Acids Res._ 31, 3429–3431 (2003) Article CAS Google Scholar * Pradhan, S. & Esteve, P. O. Allosteric activator domain of maintenance human DNA (cytosine-5) methyltransferase and its
role in methylation spreading. _Biochemistry_ 42, 5321–5332 (2003) Article CAS Google Scholar * Song, J., Rechkoblit, O., Bestor, T. H. & Patel, D. J. Structure of DNMT1-DNA complex
reveals a role for autoinhibition in maintenance DNA methylation. _Science_ 331, 1036–1040 (2011) Article ADS CAS Google Scholar * Fuerst, T. R., Niles, E. G., Studier, F. W. & Moss,
B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. _Proc. Natl Acad. Sci. USA_ 83, 8122–8126 (1986) Article ADS
CAS Google Scholar * Svedruzić, Z. M. Mammalian cytosine DNA methyltransferase Dnmt1: enzymatic mechanism, novel mechanism-based inhibitors, and RNA-directed DNA methylation. _Curr. Med.
Chem._ 15, 92–106 (2008) Article Google Scholar * Bolden, A., Ward, C., Siedlecki, J. A. & Weissbach, A. DNA methylation. Inhibition of _de novo_ and maintenance methylation _in
vitro_ by RNA and synthetic polynucleotides. _J. Biol. Chem._ 259, 12437–12443 (1984) CAS PubMed Google Scholar * Bolden, A. H., Nalin, C. M., Ward, C. A., Poonian, M. S. & Weissbach,
A. Primary DNA sequence determines sites of maintenance and _de novo_ methylation by mammalian DNA methyltransferases. _Mol. Cell. Biol._ 6, 1135–1140 (1986) Article CAS Google Scholar *
Shin, H., Liu, T., Manrai, A. K. & Liu, X. S. CEAS: _cis_-regulatory element annotation system. _Bioinformatics_ 25, 2605–2606 (2009) Article CAS Google Scholar * Heinz, S. et al.
Simple combinations of lineage-determining transcription factors prime _cis_-regulatory elements required for macrophage and B cell identities. _Mol. Cell_ 38, 576–589 (2010) Article CAS
Google Scholar * Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with RNA-Seq. _Bioinformatics_ 25, 1105–1111 (2009) Article CAS Google Scholar *
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. _Nature Protocols_ 7, 562–578 (2012) Article CAS Google Scholar
* Tsirigos, A. & Rigoutsos, I. Human and mouse introns are linked to the same processes and functions through each genome’s most frequent non-conserved motifs. _Nucleic Acids Res._ 36,
3484–3493 (2008) Article CAS Google Scholar * Bock, C. et al. Quantitative comparison of genome-wide DNA methylation mapping technologies. _Nature Biotechnol._ 28, 1106–1114 (2010)
Article CAS Google Scholar * Gill, G. & Ptashne, M. Negative effect of the transcriptional activator GAL4. _Nature_ 334, 721–724 (1988) Article ADS CAS Google Scholar *
Frank-Kamenetskii, M. D. & Mirkin, S. M. Triplex DNA structures. _Annu. Rev. Biochem._ 64, 65–95 (1995) Article CAS Google Scholar * Karreth, F. A. et al. _In vivo_ identification of
tumor-suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. _Cell_ 147, 382–395 (2011) Article CAS Google Scholar * Keene, J. D. RNA regulons: coordination of
post-transcriptional events. _Nature Rev. Genet._ 8, 533–543 (2007) Article ADS CAS Google Scholar * Figueroa, M. E. et al. DNA methylation signatures identify biologically distinct
subtypes in acute myeloid leukemia. _Cancer Cell_ 17, 13–27 (2010) Article CAS Google Scholar * Smith, Z. D., Gu, H., Bock, C., Gnirke, A. & Meissner, A. High-throughput bisulfite
sequencing in mammalian genomes. _Methods_ 48, 226–232 (2009) Article CAS Google Scholar * Xi, Y. & Li, W. BSMAP: whole genome bisulfite sequence MAPping program. _BMC Bioinformatics_
10, 232 (2009) Article Google Scholar * Benoukraf, T., Wongphayak, S., Hadi, L. H., Wu, M. & Soong, R. GBSA: a comprehensive software for analysing whole genome bisulfite sequencing
data. _Nucleic Acids Res._ 41, e55 (2013) Article CAS Google Scholar * Akalin, A. et al. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles.
_Genome Biol._ 13, R87 (2012) Article Google Scholar * Jothi, R., Cuddapah, S., Barski, A., Cui, K. & Zhao, K. Genome-wide identification of _in vivo_ protein-DNA binding sites from
ChIP-Seq data. _Nucleic Acids Res._ 36, 5221–5231 (2008) Article CAS Google Scholar * Maniatis, T., Fritsch, E. F. & Sambrook, J. _Molecular Cloning: a Laboratory Manual_ (Cold Spring
Harbor Laboratory Press, 1982) Google Scholar * Blobel, G. & Potter, V. R. Nuclei from rat liver: isolation method that combines purity with high yield. _Science_ 154, 1662–1665 (1966)
Article ADS CAS Google Scholar * Sambrook, J. & Russell, D. W. _Molecular Cloning: a Laboratory Manual_ (Cold Spring Harbor Laboratory Press, 2001) Google Scholar * Mitchell, J. A.
& Fraser, P. Transcription factories are nuclear subcompartments that remain in the absence of transcription. _Genes Dev._ 22, 20–25 (2008) Article CAS Google Scholar * Dieci, G.,
Fiorino, G., Castelnuovo, M., Teichmann, M. & Pagano, A. The expanding RNA polymerase III transcriptome. _Trends Genet._ 23, 614–622 (2007) Article CAS Google Scholar * Pagano, A. et
al. New small nuclear RNA gene-like transcriptional units as sources of regulatory transcripts. _PLoS Genet._ 3, e1 (2007) Article Google Scholar * Stewart, S. A. et al.
Lentivirus-delivered stable gene silencing by RNAi in primary cells. _RNA_ 9, 493–501 (2003) Article CAS Google Scholar * Frommer, M. et al. A genomic sequencing protocol that yields a
positive display of 5-methylcytosine residues in individual DNA strands. _Proc. Natl Acad. Sci. USA_ 89, 1827–1831 (1992) Article ADS CAS Google Scholar * Bock, C. et al. BiQ Analyzer:
visualization and quality control for DNA methylation data from bisulfite sequencing. _Bioinformatics_ 21, 4067–4068 (2005) Article CAS Google Scholar * Christman, J. K. 5-Azacytidine and
5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. _Oncogene_ 21, 5483–5495 (2002) Article CAS Google Scholar * Dunn,
J. J., Krippl, B., Bernstein, K. E., Westphal, H. & Studier, F. W. Targeting bacteriophage T7 RNA polymerase to the mammalian cell nucleus. _Gene_ 68, 259–266 (1988) Article CAS
Google Scholar * Estève, P. O. et al. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. _Genes Dev._ 20, 3089–3103 (2006) Article Google
Scholar * Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. _Nature Methods_ 5, 621–628 (2008) Article
CAS Google Scholar * Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. _Bioinformatics_ 25, 1754–1760 (2009) Article CAS Google Scholar *
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. _Nature Biotechnol._ 28, 511–515
(2010) Article CAS Google Scholar * Harrow, J. et al. GENCODE: producing a reference annotation for ENCODE. _Genome Biol._ 7, (suppl. 1)S4.1–9 (2006) Article Google Scholar * Irizarry,
R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. _Biostatistics_ 4, 249–264 (2003) Article Google Scholar * Quinlan, A. R.
& Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. _Bioinformatics_ 26, 841–842 (2010) Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by grants CA118316, CA66996 and HL56745 from the National Institutes of Health (NIH) to D.G.T., the Italian Foundation for Cancer Research (FIRC)
‘Leonino Fontana e Maria Lionello’ fellowship, the NIH T32 HL007917-11A1 and the Società Italiana di Ematologia Sperimentale (SIES) ‘Dr.Tito Bastianello’ fellowship to A.D.R.; FAMRI CIA
(103063) grant to A.K.E.; Fondazione Roma ‘Progetto cellule staminali’ to G.L. and F.D’A.; the American Italian Cancer Foundation Fellowship (AICF) to G.A.; Fundação de Amparo à Pesquisa do
Estado de São Paulo (FAPESP)—grant no. 2011/11822-6—to L.L.D.F.P.; the National Research Foundation and the Singapore Ministry of Education under its Centres of Excellence initiative to M.W.
and T.B.; the MSMT Navrat grant LK21307 to M.A.-J. S.P. and J.T. were supported by New England Biolabs. We thank R. White, D. Johnson, M. Frank-Kamenetskii, S. M. Mirkin, D. Gautheret, B.
Tazon-Vega, C. Bonifer, C. Bock, M. T. Voso, J. J. Dunn (deceased) and R. A. M. Fouchier for helpful advice and reagents; I. Rigoutsos for providing the latest released pyknons database; all
the members of the Tenen Laboratory; P. Tan and T. S. Ting from the Genome Institute of Singapore; R. Soong from the Cancer Science Institute Translational Interface; J. LaVecchio and G.
Buruzula from the Harvard Stem Cell Institute/Joslin Diabetes Center flow cytometry facility; and F. Hyde from Epicentre-Illumina. This research is supported by the Singapore Ministry of
Health’s National Medical Research Council under its Singapore Translational Research (STaR) Investigator Award (D.G.T.). AUTHOR INFORMATION Author notes * Annalisa Di Ruscio and Alexander
K. Ebralidze: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Harvard Stem Cell Institute, Harvard Medical School, Boston, 02115, Massachusetts, USA Annalisa Di
Ruscio, Alexander K. Ebralidze, Giovanni Amabile, Lorena Lobo De Figueiredo Pontes, Meritxell Alberich-Jorda, Pu Zhang, John L. Rinn & Daniel G. Tenen * Beth Israel Deaconess Medical
Center, Boston, 02115, Massachusetts, USA Annalisa Di Ruscio, Alexander K. Ebralidze, Giovanni Amabile, Lorena Lobo De Figueiredo Pontes, Meritxell Alberich-Jorda, Konstantin K. Ebralidze
& John L. Rinn * Università Cattolica del Sacro Cuore, Institute of Hematology, L.go A. Gemelli 8, Rome 00168, Italy, Annalisa Di Ruscio, Francesco D’Alò & Giuseppe Leone * Cancer
Science Institute, National University of Singapore, 117599, Singapore, Touati Benoukraf, Mengchu Wu & Daniel G. Tenen * Department of Stem Cell and Regenerative Biology, Harvard
University, 7 Divinity Avenue, Cambridge, Massachusetts 02138, USA, Loyal A. Goff & John L. Rinn * Broad Institute of MIT and Harvard, 7 Cambridge Center, Cambridge, 02142,
Massachusetts, USA Loyal A. Goff & John L. Rinn * Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, 32 Vassar Street, Cambridge,
Massachusetts 02139, USA, Loyal A. Goff * New England Biolabs, 240 County Road, Ipswich, 01938-2723, Massachusetts, USA Jolyon Terragni & Sriharsa Pradhan * Department of Pathology,
University of Michigan, Ann Arbor, 48109-2200, Michigan, USA Maria Eugenia Figueroa * Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, 142 20 Prague, Czech
Republic, Meritxell Alberich-Jorda * Department of Medicine, Hematology-Oncology, C-620 Weill Cornell Medical College, 1300 York Avenue, New York, New York 10021, USA, Ari Melnick Authors *
Annalisa Di Ruscio View author publications You can also search for this author inPubMed Google Scholar * Alexander K. Ebralidze View author publications You can also search for this author
inPubMed Google Scholar * Touati Benoukraf View author publications You can also search for this author inPubMed Google Scholar * Giovanni Amabile View author publications You can also
search for this author inPubMed Google Scholar * Loyal A. Goff View author publications You can also search for this author inPubMed Google Scholar * Jolyon Terragni View author publications
You can also search for this author inPubMed Google Scholar * Maria Eugenia Figueroa View author publications You can also search for this author inPubMed Google Scholar * Lorena Lobo De
Figueiredo Pontes View author publications You can also search for this author inPubMed Google Scholar * Meritxell Alberich-Jorda View author publications You can also search for this author
inPubMed Google Scholar * Pu Zhang View author publications You can also search for this author inPubMed Google Scholar * Mengchu Wu View author publications You can also search for this
author inPubMed Google Scholar * Francesco D’Alò View author publications You can also search for this author inPubMed Google Scholar * Ari Melnick View author publications You can also
search for this author inPubMed Google Scholar * Giuseppe Leone View author publications You can also search for this author inPubMed Google Scholar * Konstantin K. Ebralidze View author
publications You can also search for this author inPubMed Google Scholar * Sriharsa Pradhan View author publications You can also search for this author inPubMed Google Scholar * John L.
Rinn View author publications You can also search for this author inPubMed Google Scholar * Daniel G. Tenen View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS D.G.T. supervised the project; A.D.R., A.K.E. and D.G.T. conceived and designed the study; A.D.R., A.K.E., G.A., P.Z., M.A.-J., F.D’A., S.P., L.L.D.F.P. and J.T.
performed experiments; M.W. performed sequencing and microarray experiments; T.B. and L.A.G. analysed RIP-seq, RNA-seq, RRBS and microarray data; M.E.F. and A.M. performed the MassARRAY
experiment and assisted in the analysis; A.D.R., A.K.E., G.L., K.K.E., J.L.R. and D.G.T. wrote the paper. CORRESPONDING AUTHOR Correspondence to Daniel G. Tenen. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains Supplementary Results, Supplementary Methods,
Supplementary References and Supplementary Figures 1-6. (PDF 4040 kb) SUPPLEMENTARY DATA 1 This file contains the MassARRAY primer sets. (XLS 46 kb) SUPPLEMENTARY DATA 2 This file contains
a list of gene loci belonging to DNMT1-bound and unbound groups along with respective expression and DNA methylation levels. (XLS 2680 kb) SUPPLEMENTARY DATA 3 This file contains a full list
of Biological Process Gene Ontology terms significantly enriched in cluster C. (XLS 250 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR
FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Di Ruscio, A., Ebralidze, A., Benoukraf,
T. _et al._ DNMT1-interacting RNAs block gene-specific DNA methylation. _Nature_ 503, 371–376 (2013). https://doi.org/10.1038/nature12598 Download citation * Received: 13 November 2011 *
Accepted: 21 August 2013 * Published: 09 October 2013 * Issue Date: 21 November 2013 * DOI: https://doi.org/10.1038/nature12598 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative